Fig. 2.
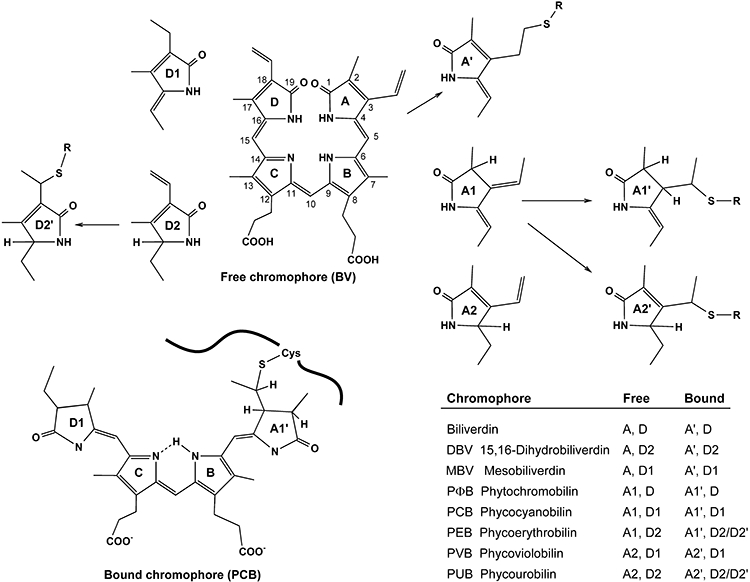
Free and protein bound bilins. Full structures of biliverdin in the typical cyclic-helical conformation of free chromophores (with IUPAC numbering, top centre), and of PCB bound at C-31 to a cysteine residue of the apoprotein, in the typical extended conformation of bound chromophores (lower left), and partial structures of modified rings A and D in other biliprotein chromophores. Rings B and C remain unchanged throughout. Protein-linked rings are marked by ‘prime’, this nomenclature is also used in the lower right table giving the names and abbreviations of the various biliprotein chromophores in the free and protein-bound form. Arrows represent lyase actions; apoprotein is indicated by heavy wavy lines.
