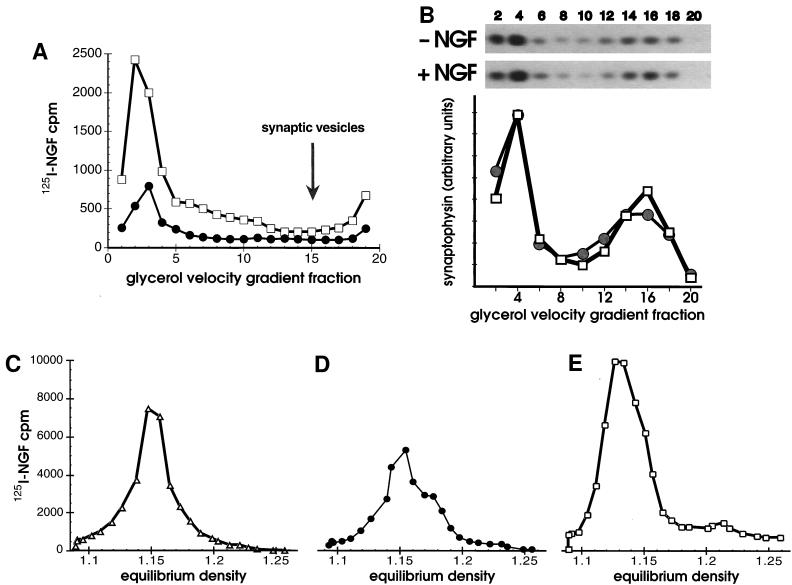
Figure 2.
(A and B) Synaptic vesicles did not contain NGF. (A) Cells bound to 125I-NGF were washed, warmed 10 min in vivo, chilled, washed, then permeabilized. Cells were fractionated by successive 1,000 × g (P1), 8,000 × g (P2), and 100,000 × g (P3) centrifugations before or after in vitro reactions with ATP. Vesicles in the P3 without an in vitro reaction, (•) or after an in vitro reaction with ATP (+ATP, □) were applied to 5–25% glycerol velocity gradients and centrifuged 200,000 × g for 1 hr. (B) Synaptophysin in cell fractions was quantified by Western blotting. After incubating with 1 nM NGF or the vehicle alone at 4°C for 1 hr, PC12 cells were warmed 10 min at 37°C, chilled, washed, and permeabilized without in vitro reactions. Glycerol velocity gradient fractions (as in A except that fractions were pooled in pairs) were analyzed by SDS/PAGE, proteins were transferred to nitrocellulose and probed with anti-synaptophysin (SY38), followed by goat anti-mouse and 125I-protein A. Autoradiograms of Western blots (above, 3 day exposure) and PhosphorImager quantification (below) show the amount of synaptophysin present in fractions in the absence of NGF (−NGF, •) or its presence (+NGF, □). The distribution of rab3A (a gift of L. Elferink and R. Scheller, Stanford University, Stanford; not shown) and synaptophysin identified synaptic vesicles in a peak at fractions 14–16. (C–E) In vitro reactions capture intermediates at different stages of endocytosis. Equilibrium density of 125I-NGF-containing vesicles in the 100,000 × g P3 (plotted on the same y-axis scale) after 2 min internalization in vivo plus a 15 min in vitro reaction with ATP (C, +ATP, ▵), or after 10 min internalization in vivo, plus a 15 min in vitro reaction without ATP (D, −ATP, •), or after 10 min internalization in vivo, plus a 15 min in vitro reaction with ATP (E, +ATP, □).