Figure 4.
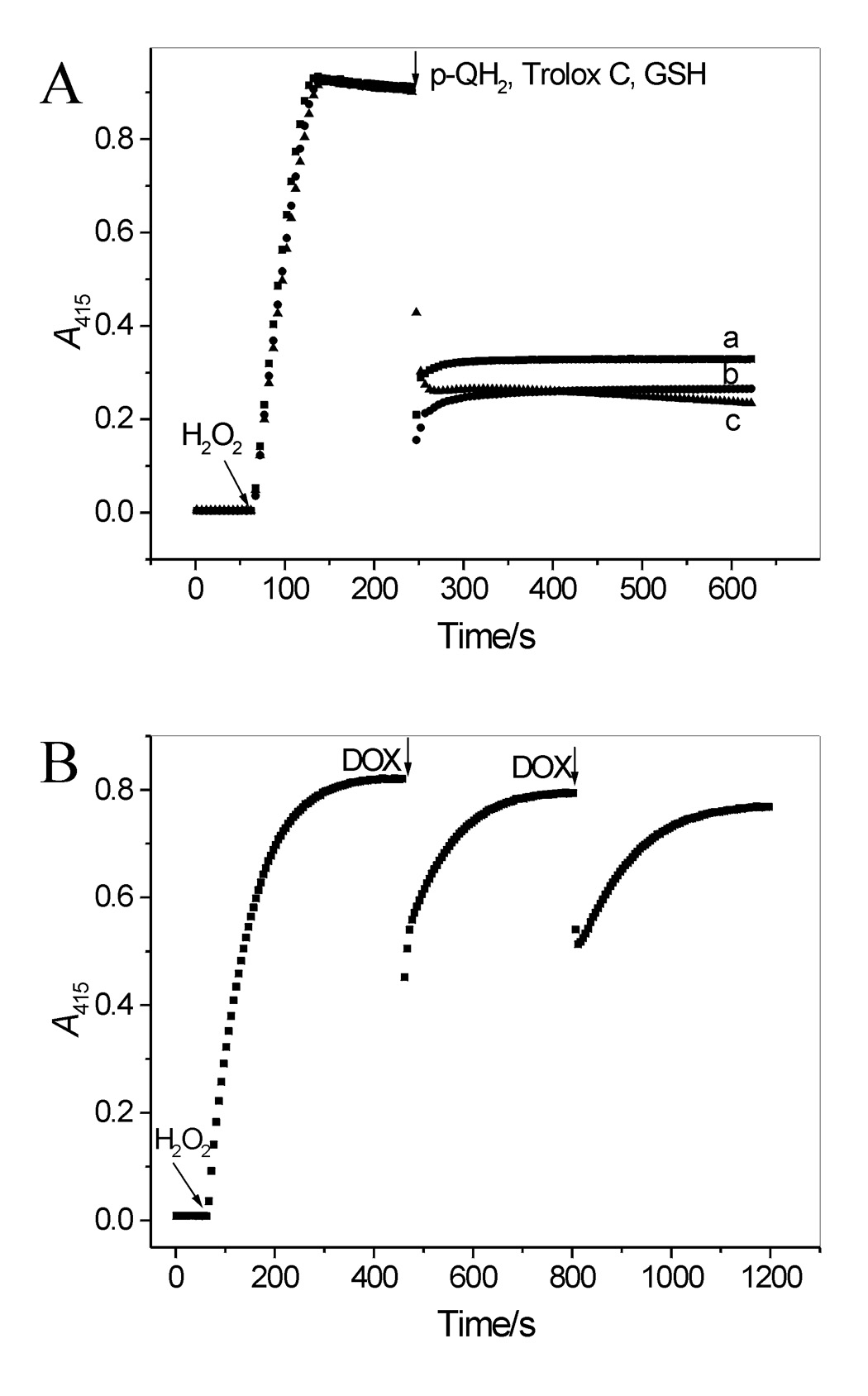
(A) Interaction of ABTS•+ with p-QH2, Trolox C and GSH. ABTS•+ was generated as described in legend to Figure 4A. When the A415 stabilized, p-QH2 was added (9.9 µM) (trace a). Similar experiments performed with Trolox C or GSH (9.9 µM each) produced traces b and c, respectively. (B) DOX reacts with ABTS•+ via electron transfer. ABTS•+ was generated by oxidizing ABTS (25 µM) by LPO and an excess of H2O2 (83 µM). When A415 stabilized, an aliquot of DOX was added (3.5 µM, two doses). After each dose, the A415 recovered, reaching ~97% of its initial level, suggesting that the transient loss of ABTS•+ was mostly due to its reduction via electron transfer from DOX(QH2). The recovered ABTS was immediately oxidized by LPO and the remaining H2O2.
