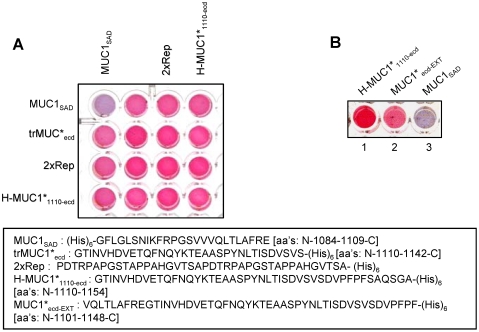
Figure 5. MUC1 has a self-aggregation domain (SAD) immediately N-terminal to MUC1*.
A. Degenerate peptides derived from the membrane-proximal extracellular domain of MUC1, as well as a portion of the tandem repeats, were separately attached to pools of nanoparticles. Aliquots of nanoparticles, each bearing a different peptide sequence, were mixed together to test for possible interaction between one peptide and another, or for self-interactions. Interactions among nanoparticle-immobilized peptides are seen as a color change from pink to purple/blue. The well in the upper left corner shows that the peptide MUC1SAD self-aggregates. No other peptides tested bound to each other. B. Wells 1–3 contain nanoparticles bearing histidine-tagged peptides of a single sequence. Well 1, containing MUC1*1110-ecd (N-1110-1154-C) does not self-aggregate as evidenced by the pink solution color. MUC1*SAD, (N-1084-1109-C), which is immediately adjacent to MUC1*1110-ecd, clearly self-aggregates as the solution color turned a deep purple/blue (Well 3). A third peptide MUC1ecd-EXT (N-1101-1148-C) comprised of 39 amino acids of MUC1*1110-ecd and extending nine additional amino acids into the self-aggregation domain (SAD), albeit to a lesser degree (Well 3).