Abstract
Current and expected changes in biodiversity have motivated major experiments, which reported a positive relationship between plant species diversity and primary production. As a first step in addressing this relationship, these manipulative experiments controlled as many potential confounding covariables as possible and assembled artificial ecosystems for the purpose of the experiments. As a new step in this endeavor, we asked how plant species richness relates to productivity in a natural ecosystem. Here, we report on an experiment conducted in a natural ecosystem in the Patagonian steppe, in which we assessed the biodiversity effect on primary production. Using a plant species diversity gradient generated by removing species while maintaining constant biomass, we found that aboveground net primary production increased with the number of plant species. We also found that the biodiversity effect was larger in natural than in artificial ecosystems. This result supports previous findings and also suggests that the effect of biodiversity in natural ecosystems may be much larger than currently thought.
Keywords: biodiversity, carbon cycle, ecosystem functioning, Patagonian steppe, resource partitioning
Human activities largely impact the natural rate of change in biodiversity by influencing species invasion, displacement, and extinction rates (1, 2). For this reason, it is crucial to understand the effects of biodiversity change on the functioning of ecosystems and their capacity for providing goods and services (1, 3). The first logical attempt to address the question of the effects of biodiversity on ecosystem functioning with an experimental approach was to create gradients of plant species richness by sowing different numbers of species into homogenized soils (4–6). These experiments with artificial ecosystems showed a positive relationship between plant species richness and productivity (4–8). A further step in our endeavor to assess the effects of biodiversity change on ecosystem functioning requires tackling this issue in natural ecosystems. Observations in natural ecosystems showed inconclusive evidence of the effect of plant species richness on productivity (4, 9–11). Manipulative experiments performed in naturally assembled communities can complement results from synthetic assemblages, which represent early successional stages (12). Here, we report an experiment designed to assess the magnitude of the plant species richness effect on aboveground net primary production (ANPP) in a natural ecosystem in the Patagonian steppe.
Our hypotheses were that increased plant species diversity would result in increased ANPP (5, 13, 14) and that the effect of biodiversity on primary production would be higher in natural than in artificial ecosystems (15). Natural ecosystems should show higher niche partitioning and stronger positive biological interactions among organisms, because species coexisted for longer periods of time and because natural ecosystems have lower frequencies of disturbance (15). Niche partitioning is the use of different resources by different species, and positive interactions are the benefits received by one species from the presence of another. On an evolutionary time scale, niche differentiation results from selection pressure and from phylogenetic constraints. On an ecological time scale, niche partitioning and positive interactions are more likely to occur when individuals are fully developed (e.g., root depth differences). Frequent disturbances will prevent the development of positive interactions and favor ruderal species (15). To test our hypotheses, we performed a manipulative experiment in the Patagonian steppe and compared the magnitude of the biodiversity effect on natural ecosystems with that on artificial ecosystems.
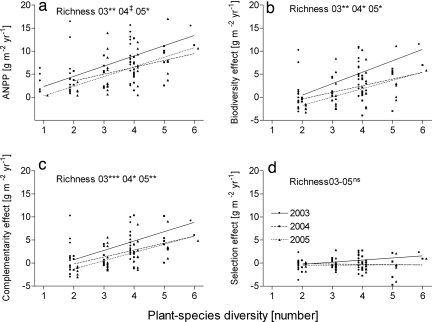
In the Patagonian steppe, we selected six target species—three grass and three shrub species—which accounted for 97% of ANPP, 94% of aerial cover [supporting information (SI) Table 2], and 10% of the total number of plant species in the steppe (16). We created a plant species richness gradient by removing plant species and portions of individuals. By removing plant species, we changed the original species number of the experimental units to generate a gradient of one, two, four, and six species, with all possible assemblages replicated; by removing portions of individuals, we obtained equal biomass along the species gradient (SI Fig. 3). Biomass of target species at the beginning of the experiment was the same for all experimental 5 × 5 m plots (SI Fig. 3A). In addition, total removed and remaining biomasses of target species were the same across all of the treatments (SI Fig. 3 B and C). Our results support the biodiversity-productivity hypothesis (13) that states that increases in plant species richness results in increases in ANPP (Fig. 1a and Table 1). We found a linear and positive relationship between ANPP and plant species richness (observed at the time of ANPP estimate) for three growing seasons. Meristem density increased with species richness (Table 1 and SI Fig. 4), and tussock area increased with grass species richness (Table 1 and SI Fig. 5). Both results provided mechanisms at the individual-plant level by which increasing the number of species affected productivity.
Fig. 1.
Relationship between aboveground net primary production (ANPP) (a), biodiversity effect (b), complementarity effect (c), and selection effect and plant species richness (d) in the Patagonian steppe. During 3 years from 2003 to 2005 ANPP, the biodiversity (21) and complementarity (21) effects increased linearly with observed plant species richness, whereas the selection effect (21) remained constant. We estimated the biodiversity effect for a mixture as the difference between observed and expected ANPP (the yield in monoculture weighted by the initial proportion of species in the mixture), using the equation proposed by Loreau and Hector (21). We unpacked the biodiversity effect by calculating its two components, selection and complementarity effects. Each point represents the average of an assemblage, and lines represent best fit. In all cases, the assemblage term was not significant. See Table 1 for statistical analysis. ‡, P < 0.07; *, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, P > 0.1.
Table 1.
Summary of statistical analyses performed between biodiversity measured as total plant-species richness and grass-species richness and ANPP, the biodiversity effect and its components, niche complementarity, and selection effect (21)
| Variable | yr | Richness |
Assemblage |
||||
|---|---|---|---|---|---|---|---|
| df | MS | F | df | MS | F | ||
| Plant-species richness | |||||||
| ANPP | 2003 | 1 | 274.7 | 7.9** | 35 | 34.7 | 1.27 |
| 2004 | 1 | 43.4 | 3.6‡ | 26 | 12.0 | 0.9 | |
| 2005 | 1 | 66.0 | 4.0* | 32 | 16.5 | 1.2 | |
| biodiversity effect | 2003 | 1 | 337.8 | 11.0** | 29 | 30.7 | 0.9 |
| 2004 | 1 | 47.3 | 4.3* | 25 | 11.1 | 0.7 | |
| 2005 | 1 | 83.6 | 6.3* | 32 | 13.3 | 1.0 | |
| niche complementarity | 2003 | 1 | 370.2 | 12.9*** | 29 | 28.8 | 1.3 |
| 2004 | 1 | 41.3 | 4.9* | 25 | 8.4 | 0.9 | |
| 2005 | 1 | 93.0 | 10.0** | 32 | 9.3 | 1.7‡ | |
| selection effect | 2003 | 1 | 1.9 | 0.2 | 29 | 8.5 | 1.1 |
| 2004 | 1 | 0.2 | 0.5 | 25 | 4.2 | 1.0 | |
| 2005 | 1 | 0.3 | 0.2 | 32 | 1.7 | 0.5 | |
| meristem density | 2003 | 1 | 0.353 | 7.53*** | 35 | 0.047 | 1.91* |
| 2004 | 1 | 0.317 | 4.75* | 35 | 0.067 | 2.04* | |
| Grass species richness | |||||||
| grass area | 2003 | 1 | 0.185 | 14.9* | 5 | 0.01 | 0.73 |
| NO3-N | May 2003 | 1 | 1.81 | 4.41* | 35 | 0.40 | 0.85 |
| NH4-N | Oct 2002 | 1 | 85.5 | 3.89‡ | 35 | 21.9 | 0.83 |
The table also reports statistical analyses of the effects of species richness on meristem density, area of individual grasses, and soil nitrate and ammonium. The generalized linear model showed that ANPP, biodiversity effect, niche complementarity, and meristem density (SI Fig. 4) all increased with increased plant-species richness and that the basal area of grasses (SI Fig. 5) increased with increased grass-species richness, whereas soil inorganic nitrogen (SI Fig. 7) decreased with grass-species richness. The selection effect was not affected by richness or the composition of species.
yr, year; df, degree of freedom; MS, mean square; F, Fisher statistic.
‡, P < 0.07;
*, P < 0.05;
**, P < 0.01;
***, P < 0.001.
The observed pattern of increase in ANPP with plant species richness can be explained by nonexclusive mechanisms: (i) complementary use of resources among species; (ii) positive interactions among species (17, 18); and (iii) the “sampling effect,” which results from the higher probability of including the most productive species in higher diversity mixtures (19, 20). Together, these mechanisms constitute the biodiversity effect, which is the portion of the ANPP that results from increased biodiversity; the effect can be analytically estimated as the difference between the observed and expected yields of species mixtures (21). Expected ANPP for a mixture is the sum of the productivity of each species in monocultures, weighted by the initial proportion of species in the mixture (21).
We found that the biodiversity effect increased linearly with species richness (Fig. 1b and Table 1). The proportion of ANPP accounted for by the biodiversity effect (21) increased with species richness and had an average 64% highest diversity in three growing seasons. Increases in biodiversity effect were mainly derived from increases in the complementarity effect (21) (Fig. 1 b and c and Table 1). Resources used by plants supported the idea of niche complementarity. We found that plots with grasses, which in the Patagonian steppe are shallow-rooted in comparison with shrubs (22), decreased soil water content on the upper 20 cm of the soil profile in spring and at the end of the growing season (ANOVA: Oct 02, F1,82 = 4.095, P < 0.05; May 03, F1,82 = 3.620, P < 0.061) (SI Fig. 6). Similarly, grass richness decreased inorganic N availability on the top soil layer (Table 1 and SI Fig. 7). The selection effect, a general form of the sampling effect (21) component remained constant along the gradient of species richness and close to zero (Fig. 1d and Table 1).
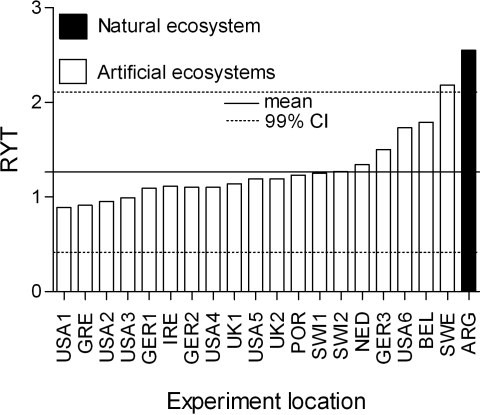
We compared natural and artificial ecosystems with the relative yield total (RYT) (23), an index that quantifies the performance of a species growing in a mixture relative to its monoculture. We generated a normal distribution with values of RYT reported for artificial ecosystems (see SI Table 3 for the list of experiments included), and compared the natural ecosystem against a 99% confidence interval (CI). The RYT in Patagonia was higher than the upper limit of the CI (Fig. 2).
Fig. 2.
Effect of plant species richness on aboveground net primary production (ANPP) in natural and artificial ecosystems. The relative yield total (RYT) is the ratio between species ANPP in mixtures (Oi) and its ANPP in monocultures (Mi), summed for all species in the mixture (N) (RYT = Σi=1N Oi/Mi) (23); we used a corrected version of the RYT (SI Text, RYT). Bars represent the RYT for the maximum richness level averaged among years for different experiments conducted at different localities. Artificial ecosystems were created by sowing different numbers of species in pots or field plots with homogenized soils (i.e., chemically sterilized soils) for the sake of gaining control of the experiment (12); in contrast, the experiment conducted in the natural ecosystem reported here was created by removing species and portions of individuals from established communities in the Patagonian steppe. Dotted lines represent a confidence interval of 99% around the average of artificial-ecosystems. Codes used are as follows: USA1 and USA3, Texas; USA2, North Carolina; USA4, California; USA5 and USA6, Minnesota; GRE, Greece; GER1, Bayreuth, Germany; GER2 and GER3, Jena, Germany; IRE, Ireland; UK1, Silwood Park, United Kingdom; UK2, Sheffield, United Kingdom; POR, Portugal; SWI1, Zürich; SWI2, Lupsingen, Switzerland; NED, The Netherlands; BEL, Belgium; SWE, Sweden; and ARG, Argentina (see SI Table 3 for references).
In the natural ecosystem in Patagonia, biodiversity accounted for a higher proportion of ANPP than in artificial ecosystems, probably because of large species differences in resource use and positive interactions among species. In the Patagonian experiment, niche partitioning and positive interactions reflected long-term species coexistence. Grasses and shrubs in the steppe showed extensive niche partitioning for soil water use with depth (22, 24). Other differences are also very likely to occur, such as soil water use in time by species with different phenology, and differences in nutrient use in space and time (25). In Patagonia, facilitation had been shown between established shrubs and grass seedlings (26) and between a legume, Adesmia campestris, and a grass, Stipa humilis (27).
In artificial ecosystems, both niche complementarity and facilitation may have been reduced by (i) the use of seeds from external sources (6, 28),which reduced the possibility of local adaptation (29, 30); (ii) the use of naturalized species (31), which decreased the probability of finding long-term biotic interactions that could result in niche partitioning either by character displacement (32) or in positive interactions (33); (iii) a high disturbance frequency, such as fire or mowing, which limited the establishment of positive interactions among species (15); and (iv) conducting relatively short-duration experiments that did not allow full development of roots and shoots in long-lived plant species and that consequently may have reduced partitioning of soil resources. In contrast, natural ecosystems presented mature individuals, populations, and species coexisting for long periods of time in natural soils without chemical treatments and low artificial disturbance regimes.
We found that plant species richness had higher effects on productivity in natural ecosystems than has been shown in numerous experiments using artificial ecosystems. This larger effect of biodiversity on ecosystem functioning suggests that the consequences of biodiversity loss at the global scale may have been severely underestimated. The ability of ecosystems to cope with global change and the ability of our planet to maintain life-supporting mechanisms may be constrained by continuing losses of species diversity. For example, the capacity of dominant natural ecosystems to sequester carbon emitted by humans might be limited by the simultaneous loss of biodiversity, which also results from human activity.
Methods
Study Site.
The experiment was conducted in the Patagonia steppe, near the town of Río Mayo, Argentina (lat 45°41′S, long 70°16′W). The climate is semiarid, with 170 mm of annual precipitation occurring mostly during fall and winter. Average monthly temperatures range from 2°C in winter to 14°C in summer. Soils are coarse textured with gravel and stones. Vegetation corresponds to the Occidental floristic district (34) and is dominated by three grass species, Stipa speciosa Trin et Ruprecht, S. humilis Cav., and Poa ligularis Nees ap. Steud, and three shrub species, Mulinum spinosum (Cav.) Pers., A. campestris (Rendle) Rowlee, and Senecio filaginoides DC.
Experimental Design.
Using the six target species mentioned above, we generated a gradient of one, two, four, and six species, replicating all possible assemblages. Our design included the 6, 15, 15, and 1 possible assemblage, each of them replicated three, two, two, and six times for the one, two, four, and six species diversity levels, respectively. To create the gradient, we first selected 84 5 × 5 m plots with the same biomass and all six target species (SI Fig. 3A), then we randomly assigned biodiversity treatments to plots. To implement treatments, first we removed species from the plots and left only target species, then we removed portions of individuals with wedges of different angles to equalize the biomass to the average biomass of monocultures (SI Text, Experimental Design). In this way, our removal disturbance was the same among all plots (SI Fig. 3B) and resulted in a species richness gradient that initially had similar biomass (SI Fig. 3C; P > 0.05) but a different number of species (SI Text, Experimental Design). Other plant species, different from the six target species, represented <2.5% of total cover and 52% of the total average number of species in a 5 × 5 m plot; non target species were removed at the beginning of the experiment.
Response Variables.
We estimated aboveground net primary production as the difference between aboveground biomass in autumn and in summer. To estimate biomass, we used a nondestructive method in which we measured plant species cover and transformed those values, using calibration curves (35). We estimated the biodiversity effect, and both of its components, the selection and the complementarity effects, using the equations proposed by Loureau and Hector (21). The biodiversity effect is the difference between the observed and expected ANPP in a mixture of species (21). The expected ANPP is estimated by using the initial proportion of species in a mixture and the average production of the species in monoculture. The biodiversity effect can be divided into its two components: (i) the selection effect, which is the portion of increase in production with increasing diversity that results from the increased probability of including in the mixture the species with highest individual performance; and (ii) the complementarity effect, which is the portion of the change in production with increasing diversity that results from the differences in resource utilization among species in a mixture. To estimate the biodiversity effect and relative yield total (RYT) in 2004 and 2005, we used the monoculture yields for 2003, because the number of species in single-species plots increased after the first growing season, and we did not find differences in monoculture ANPP among years (P > 0.05).
We estimated meristem density at the time of peak green biomass. We did so by randomly choosing one individual per species present in a plot in which we counted, for grasses, the number of tillers per tussock-basal-area, and, for shrubs, the number of twigs in 250 cm2. For each species, the value of meristem density was rescaled to its maximum to average them with other species per plot. In this way, meristem density reflects the response of a plant individual irrespective of the species in a plot. We used the difference in tussock-grass basal area to estimate plant-individual responses. We estimated volumetric soil water content from 0- to 25-cm depth with the time domain reflectometry technique (Tecktronix 1502C), from spring to autumn. We estimated inorganic soil N, using 2 N ClK extracts that we analyzed with an Alpkem (O-I Corporation).
Statistical Analysis.
We used a general linear model with plant and grass species richness as a continuous variable and assemblage nested within richness to evaluate the effects on the variables listed above. The richness term evaluated the effect of the number of species, whereas the assemblage term evaluated the effect of the composition of species. The F test to evaluate the species richness term was constructed with the mean square (MS) of species richness divided by the MS of assemblage. To evaluate the assemblage term, we divided the MS of assemblage by the MS of the unexplained variance (6). We checked for normal distribution of errors. The only exception to this analysis was soil water content, for which we performed an ANOVA comparing plots with and without grasses. To compare the artificial with the natural ecosystems, we tested the value of the relative yield total obtained for Patagonia against a confidence interval of 2.576 standard deviations (99%) around the average values for artificial ecosystems.
Artificial Ecosystems.
Here, we defined artificial ecosystems as those in which the experimental community and species composition started from seeds planted by researchers. In contrast, experiments in natural ecosystems started with original communities that were manipulated to create a gradient of species richness. We constrained our analysis to experiments that (i) manipulated the number of species; (ii) had all possible monocultures; (iii) used vascular plants in terrestrial ecosystems; (iv) had more than two species; (v) were field experiments; and (vi) focused on the effect of biodiversity on productivity. To compare experiments, we used the RYT for the maximum level of diversity. In experiments with manipulation of nutrient or CO2, we only included control treatments (SI Table 3).
Supplementary Material
Acknowledgments.
We thank A. T. Austin, M. L Yahdjian, L. Vivanco, L. G. Reichmann, C. Shufro, M. Bertness, J. B. H. Martiny, and two anonymous reviewers for their suggestions during the experiment and manuscript preparation; D. Tilman, P. Reich, and J. Dukes for sharing their experimental data; and J. Vrsalovic, M. Covalschi, L. Covalschi, M. Gonzalez Polo, and those who collaborated in the field. This work was supported by the Institute for Agricultural Plant Physiology and Ecology, the Agricultural Research Service of Argentina of Río Mayo, the Argentina National Council for Research, the Argentina Agency for Research and Technology, the Inter-American Institute for Global Change Research, the University of Buenos Aires, and Brown University.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0704801105/DC1.
References
- 1.Millennium Ecosystem Assessment. Ecosystems and Human Well-Being: Synthesis. Washington, DC: Island; 2005. [Google Scholar]
- 2.Sala OE, et al. Global biodiversity scenarios for the year 2100. Science. 2000;287:1770–1774. doi: 10.1126/science.287.5459.1770. [DOI] [PubMed] [Google Scholar]
- 3.Chapin FS, et al. Consequences of changing biodiversity. Nature. 2000;405:234–242. doi: 10.1038/35012241. [DOI] [PubMed] [Google Scholar]
- 4.Tilman D, Wedin D, Knops J. Productivity and sustainability influenced by biodiversity in grassland ecosystems. Nature. 1996;379:718–720. [Google Scholar]
- 5.Naeem S, Thompson LJ, Lawler SP, Lawton JH, Woodfin RM. Declining biodiversity can alter the performance of ecosystems. Nature. 1994;368:734–737. [Google Scholar]
- 6.Hector A, et al. Plant diversity and productivity experiments in European grasslands. Science. 1999;286:1123–1127. doi: 10.1126/science.286.5442.1123. [DOI] [PubMed] [Google Scholar]
- 7.Hooper DU, et al. Effects of biodiversity on ecosystem functioning: A consensus of current knowledge. Ecol Monogr. 2005;75:3–35. [Google Scholar]
- 8.Cardinale BJ, et al. Effects of biodiversity on the functioning of trophic groups and ecosystems. Nature. 2006;443:989–992. doi: 10.1038/nature05202. [DOI] [PubMed] [Google Scholar]
- 9.Grace JB, et al. Does species diversity limit productivity in natural grassland communities? Ecol Lett. 2007;10:680–689. doi: 10.1111/j.1461-0248.2007.01058.x. [DOI] [PubMed] [Google Scholar]
- 10.Thompson K, Askew AP, Grime JP, Dunnett NP, Willis AJ. Biodiversity, ecosystem function and plant traits in mature and immature plant communities. Funct Ecol. 2005;19:355–358. [Google Scholar]
- 11.Loreau M, et al. Biodiversity and ecosystem functioning: Current knowledge and future challenges. Science. 2001;294:804–808. doi: 10.1126/science.1064088. [DOI] [PubMed] [Google Scholar]
- 12.Díaz S, Symstad AJ, Chapin FS, Wardle DA, Huenneke LF. Functional diversity revealed by removal experiments. Trends Ecol Evol. 2003;18:140–146. [Google Scholar]
- 13.Vitousek PM, Hooper DU. In: Biodiversity and Ecosystem Function. Schulze ED, Mooney HA, editors. Berlin: Springer-Verlag; 1993. pp. 3–14. [Google Scholar]
- 14.Schläpfer F, Schmid B. Ecosystem effects of biodiversity: A classification of hypotheses and exploration of empirical results. Ecol Appl. 1999;9:893–912. [Google Scholar]
- 15.Sala OE. Price put on biodiversity. Nature. 2001;412:34–36. doi: 10.1038/35083676. [DOI] [PubMed] [Google Scholar]
- 16.Golluscio RA, Leon RJC, Perelman S. Caracterización fitosociológica del oeste de Chubut, su relación con el gradiente ambiental. Boletín Sociedad Argentina Botánica. 1982;21:299–324. [Google Scholar]
- 17.Tilman D, et al. The influence of functional diversity and composition on ecosystem processes. Science. 1997;277:1300–1302. [Google Scholar]
- 18.Loreau M. Biodiversity and ecosystem functioning: A mechanistic model. Proc Natl Acad Sci USA. 1998;95:5632–5636. doi: 10.1073/pnas.95.10.5632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huston MA. Hidden treatments in ecological experiments: Re-evaluating the ecosystem function of biodiversity. Oecologia. 1997;110:449–460. doi: 10.1007/s004420050180. [DOI] [PubMed] [Google Scholar]
- 20.Tilman D, Lehman C, Thomson K. Plant diversity and ecosystem productivity: Theoretical considerations. Proc Natl Acad Sci USA. 1997;94:1857–1861. doi: 10.1073/pnas.94.5.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loreau M, Hector A. Partitioning selection and complementarity in biodiversity experiments. Nature. 2001;412:72–76. doi: 10.1038/35083573. [DOI] [PubMed] [Google Scholar]
- 22.Sala OE, Golluscio RA, Lauenroth WK, Soriano A. Resource partitioning between shrubs and grasses in the Patagonian steppe. Oecologia. 1989;81:501–505. doi: 10.1007/BF00378959. [DOI] [PubMed] [Google Scholar]
- 23.de Wit R, van den Bergh JP. Competition between herbage plants. Netherlands J Agr Sci. 1965;13:212–221. [Google Scholar]
- 24.Schulze ED, et al. Rooting depth, water availability, and vegetation cover along an aridity gradient in Patagonia. Oecologia. 1996;108:503–511. doi: 10.1007/BF00333727. [DOI] [PubMed] [Google Scholar]
- 25.McKane RB, et al. Resource-based niche provide a basis for plant species diversity and dominance in Artic tundra. Nature. 2002;415:68–71. doi: 10.1038/415068a. [DOI] [PubMed] [Google Scholar]
- 26.Aguiar MR, Sala OE. Competition, facilitation, seed distribution and the origin of patches in a Patagonian steppe. Oikos. 1994;70:26–34. [Google Scholar]
- 27.Soriano A, Sala OE, Perelman SB. Patch structure and dynamics in a Patagonian arid steppe. Vegetatio. 1994;111:127–135. [Google Scholar]
- 28.Roscher C, et al. Overyielding in experimental grassland communities—irrespective of species pool or spatial scale. Ecol Lett. 2005;8:419–429. [Google Scholar]
- 29.Kawecki TJ, Ebert D. Conceptual issues in local adaptation. Ecol Lett. 2004;7:1225–1241. [Google Scholar]
- 30.Joshi J, et al. Local adaptation enhances performance of common plant species. Ecol Lett. 2001;4:536–544. [Google Scholar]
- 31.Reich PB, et al. Plant diversity enhances ecosystem responses to elevated CO2 and nitrogen deposition. Nature. 2001;410:809–812. doi: 10.1038/35071062. [DOI] [PubMed] [Google Scholar]
- 32.Brown WL, Wilson EO. Character displacement. Syst Zool. 1956;5:49–64. [Google Scholar]
- 33.Bruno JF, Stachowicz JJ, Bertness MD. Inclusion of facilitation into ecological theory. Trends Ecol Evol. 2003;18:119–125. [Google Scholar]
- 34.Soriano A, Sala OE. Ecological strategies in a Patagonian arid steppe. Vegetatio. 1983;56:9–15. [Google Scholar]
- 35.Flombaum P, Sala OE. A non-destructive and rapid method to estimate biomass and aboveground net primary production in arid environments. J Arid Environ. 2007;69:352–358. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.