Fig. 4.
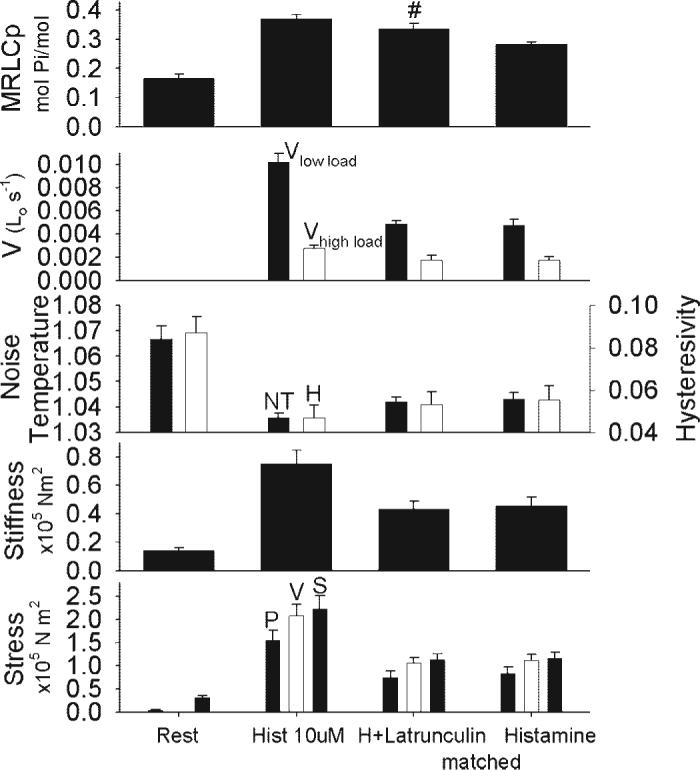
Mean biochemical and mechanical characteristics in swine carotid artery tissues with and without latrunculin-A-induced actin disruption. Tissues were either unstimulated (column 1), maximally stimulated with 10 μM histamine for 25−40 min (column 2), stimulated with 10 μM histamine and relaxed with 6 μM latrunculin-A to ∼50% of maximal force (column 3), or stimulated with various concentrations of histamine to the same ∼50% of maximal force (column 4). The panels shown mean ± 1 SE for crossbridge (MRLC) phosphorylation (panel 1), shortening velocity at low load (panel 2, filled bars labeled Vlow load), shortening velocity at high load (panel 2, open bars labeled Vhigh load), noise temperature (panel 3, filled bars), hysteresivity (panel 3, open bars), stiffness (panel 4), and stress (panel 5, filled bars on left are the stress from the MRLC phosphorylation experiments, the open bars are the stress from the velocity experiments, and the filled bars on right are from the stiffness experiments). Data are presented as mean ± 1 SE with n = 4−6 experiments. #Significant difference when comparing tissues with and without actin disruption, i.e., column 3 vs. column 4. Latrunculin-A treatment was associated with higher crossbridge phosphorylation when compared to tissues without latrunculin-A treatment at the same force. Velocity, noise temperature, hysteresivity, and stiffness did not significantly differ with and without latrunculin-A treatment.
