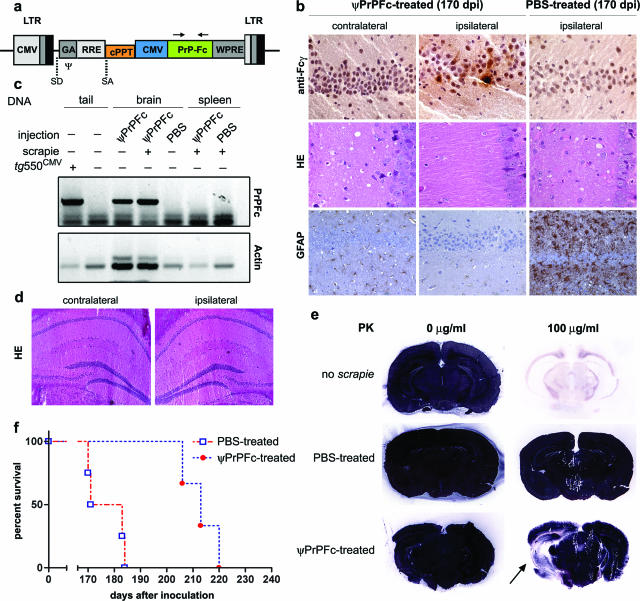
Figure 1.
Intrahippocampal lentiviral transfer of PrP-Fc2. a: Schematic representation of the transfer vector used in this study. b: Hippocampal PrP-Fc2 expression in the injected hippocampus (top row) coincided with suppressed prion pathogenesis in ψPrPFc-treated mice sacrificed at 170 dpi (middle and bottom rows), as assessed by immunohistochemistry using an anti-human Fcγ antibody. c: PCR using primers indicated in a of various tissues and genotypes showing brain-specific lentiviral integration. Genomic actin amplification served for normalization of the PrPFc signal (concentration of genomic DNA in crude extract from the tissue samples was not controlled in this experiment). d: No pathology in the brain was associated with the lentiviral injection as determined by H&E staining and by various markers (GFAP, CD11b, Iba-1; data not shown) in nonprion inoculated mice. e: Histoblots showed a broad area of decreased PrPSc deposition extending well beyond the site of ψPrPFc injection (arrow). In contrast, PrPSc signals were saturated in the contralateral hemisphere and in a PBS-treated brain. f: Survival plots displaying the incubation time until development of terminal scrapie in PBS- and ψPrPFc-treated mice after intracerebral challenge. Median disease onset was delayed by 36 days in intrahippocampally ψPrPFc-treated mice. LTR, long terminal repeats; CMV, cytomegalovirus; SD, splice donor; SA, splice acceptor; Ψ, packaging sequence; GA, 5′ portion of gag gene with truncated reading frame including extended packaging signal; RRE, rev response element (RRE); cPPT, central polypurine tract; WPRE, posttranscriptional regulatory element from the genome of the woodchuck hepatitis virus.