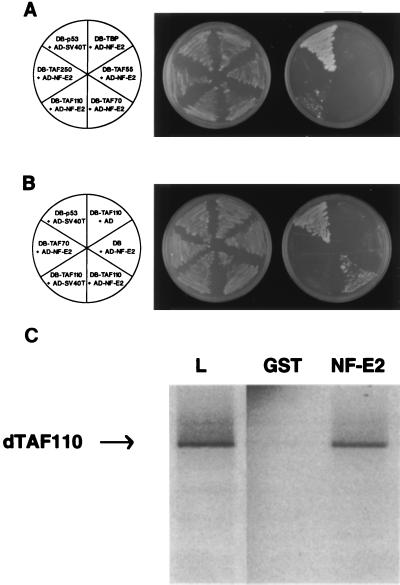
Figure 2.
Human NF-E2 interacts specifically with dTAFII110. (A) The yeast two-hybrid assay demonstrates that p45NF-E2 interacts with dTAFII110. The Saccharomyces cerevisiae reporter strain HF7C was transformed with the indicated plasmids. AD-NF-E2 contains the entire coding sequence of human NF-E2 inserted in-frame with the activation domain of GAL4 (amino acids 768–881). DB-TBP, DB-TAFII55, DB-TAFII70, and DB-TAFII250 contain the coding sequence of the human proteins and DB-TAFII110 the coding sequence of the Drosophila protein inserted in-frame to the GAL4 DNA binding domain (amino acids 1–147). A specific interaction between DB-p53 and AD-simian virus 40 T antigen has been reported previously. Leu- and Trp- transformants were streaked onto synthetic medium plates lacking tryptophan, leucine, and histidine to assess potential interactions (Right) and synthetic medium plates lacking tryptophan and leucine to confirm plating efficiency (Middle). The plates were incubated at 30°C for 3 days. (B) The yeast two-hybrid assay demonstrates that the NF-E2/dTAFII110 interaction is specific. DB-TAF110 was plated in combination with the simian virus 40 T antigen or GAL4-AD. AD-NF-E2 was plated in combination with TAFII110, TAFII70, or the GAL4-DB. Transfections were grown in the absence of leucine and tryptophan to assess transformation efficiency (Middle) or in the absence of leucine, tryptophan and histidine to assess protein-protein interactions (Right). Cotransfection of AD-simian virus 40 T-antigen and DB-p53 served as the positive control. (C) GST chromatography confirms that dTAFII110 binds specifically to p45NF-E2. One microgram of GST or GST-NF-E2, bound to glutathione-Sepharose was incubated with in vitro-translated dTAFII110 as described in Materials and Methods. Bound proteins were detected by SDS/PAGE followed by autoradiography. An example of labeled dTAFII110 that was loaded is shown in lane L.