A novel regulator of antibiotic production in S. coelicolor, AbsC, has been crystallized in space group P212121. X-ray data to 2.25 Å resolution were collected on station PX 14.1 at Daresbury.
Keywords: AbsC, Streptomyces, antibiotic production, MarR homologue, transcriptional regulation
Abstract
Crystals of recombinant AbsC (subunit MW = 18 313 Da; 158 amino acids), a novel regulator of antibiotic production from Streptomyces coelicolor, were grown by vapour diffusion. The protein crystallizes in space group P212121, with unit-cell parameters a = 43.53, b = 121.30, c = 143.75 Å. Native data to a resolution of 2.25 Å were recorded at station PX 14.1 (Daresbury) from a single crystal. Preliminary analysis of these data suggests that the asymmetric unit contains four copies of the AbsC monomer, giving an estimated solvent content of 47.0%. AbsC belongs to the MarR family of proteins that mediate ligand-responsive transcriptional control.
1. Introduction
In order to survive and compete effectively, bacteria must continuously monitor both their surroundings and their physiological status and react appropriately (Camilli & Bassler, 2006 ▶). The resultant cellular responses are frequently triggered by the binding of small signalling molecules to regulatory proteins. The activities of the MarR family of transcriptional regulators are thought to be modulated in this way (Wilkinson & Grove, 2006 ▶). These cytoplasmic proteins control a variety of biological functions in bacteria, including antibiotic resistance (Miller & Sulavik, 1996 ▶; Alekshun et al., 2001 ▶), the response to oxidative stress (Spory et al., 2002 ▶; Wilkinson & Grove, 2004 ▶) and the synthesis of pathogenic factors (Rouanet et al., 2004 ▶; Wyborn et al., 2004 ▶), and thus they are of significant clinical interest. They exist in solution as homodimers and control gene expression by interacting with palindromic (or pseudopalindromic) DNA sequences through conserved winged-helix DNA-binding motifs (Wilkinson & Grove, 2006 ▶). Although the structures of several MarR family members are now known (Wilkinson & Grove, 2006 ▶), only the MarR structure has been determined with a bound ligand, namely salicylate (Alekshun et al., 2001 ▶), and only the OhrR structure has been determined bound to DNA (Hong et al., 2005 ▶). The absC gene (antibiotic synthesis deficient) encodes a novel pleiotropic regulator of antibiotic synthesis in Streptomyces coelicolor that belongs to the MarR family (Kock, Mootien & Bibb, unpublished results). We report here the crystallization and preliminary X-ray analysis of the AbsC protein from S. coelicolor as part of a multidisciplinary study towards understanding its physiological role.
2. Materials and methods
2.1. Protein expression and purification
The absC-coding sequence (ScoDB accession No. SCO5405; http://streptomyces.org.uk/sco/index.html) was amplified by PCR with S. coelicolor M145 chromosomal DNA as the template and using primers containing an NdeI restriction site in the forward primer (5′-GGAATTCCATATGGAGACCGAGACGGCC-3′) and a BamHI site in the reverse primer (5′-CGGGATCCTCAGGGTCGTCCCCGCT-3′). The amplified DNA was subsequently digested with NdeI and BamHI and cloned into the NdeI/BamHI-digested expression vector pET15b (Novagen) to yield plasmid pET15b-absC-M145, encoding the AbsC protein with a thrombin-cleavable N-terminal hexahistidine tag. This added a further 20 residues to the native protein (with sequence MGSSHHHHHHSSGLVPRGSH), giving a total deduced molecular weight of 20 476 Da. pET15b-absC-M145 was then introduced into BL21(DE3) (pLysS) cells by transformation (Studier & Moffatt, 1986 ▶). For protein expression, 10 ml of an overnight culture of these cells was used to inoculate 1 l Luria–Bertani medium (without glucose) containing 100 mg ampicillin and 30 mg chloramphenicol. The cells were grown at 310 K to an OD600 of around 0.4. Protein expression was induced by the addition of isopropyl β-d-thiogalactopyranoside to a final concentration of 1 mM and the culture was shaken for an additional 6 h at 310 K. The cells were harvested by centrifugation in a Sorvall Evolution centrifuge (10 min, 7000 rev min−1, 277 K, SLC-6000 rotor) and stored at 253 K until further purification.
The cell pellet was resuspended in buffer A (50 mM phosphate pH 7.8, 300 mM NaCl, 5 mM imidazole) containing a Complete EDTA-free protease-inhibitor cocktail (Roche) and lysed by two passes through a French press (6.9 MPa, 277 K). The cell debris was removed by centrifugation in a Sorvall RC5C centrifuge (30 min, 20 000 rev min−1, 277 K, SS34 rotor) and the supernatant was applied onto a pre-equilibrated 5 ml Ni2+-charged His-Trap Chelating HP column (GE Healthcare). The column was then washed with buffer A to remove any unbound proteins and the bound protein was eluted in a linear gradient to 750 mM imidazole in buffer A. The fractions containing the AbsC protein (confirmed by SDS–PAGE) were pooled and concentrated to approximately 10 mg ml−1 using an Amicon Ultra-4 10 kDa cutoff concentrator (Millipore). To further purify the protein for crystallization, the protein was applied onto a Superdex 75 HiLoad HP gel-filtration column (GE Healthcare) pre-equilibrated with 50 mM phosphate pH 7.8, 300 mM NaCl, 10 mM EDTA, 10 mM DTT which had previously been calibrated using low-molecular-weight protein standards (GE Healthcare). The elution fractions containing AbsC were pooled and concentrated to around 10 mg ml−1 using an Amicon Ultra-4 10 kDa cutoff concentrator (Millipore). The N-terminal His tag was not cleaved from the protein sample.
Prior to crystallization, dynamic light scattering (DLS) was used to monitor the solution properties of the purified protein. For this purpose, approximately 30 µl of sample was centrifuged through a 0.1 µm Ultrafree-MC filter (Millipore) to remove particulate material before introduction into the 12 µl microsampling cell of a DynaPro-MSTC molecular-sizing instrument (Protein Solutions Inc.). A minimum of 20 scattering measurements were taken at 277 and 293 K and the resulting data were analysed using the DYNAMICS software package (Protein Solutions Inc.).
2.2. Crystallization and X-ray data collection
Crystallization screening trials were carried out by vapour diffusion in a sitting-drop format in 96-well Greiner plates using a variety of in-house and commercially available screens (Hampton Research, Molecular Dimensions and Nextal) at a constant temperature of 293 K. Drops consisted of 1 µl protein solution mixed with 1 µl well solution and the well volume was 50 µl. The protein concentration was approximately 10 mg ml−1. Several conditions gave crystals in the initial screens and improved crystals were subsequently obtained by refining these successful conditions and adapting them to a hanging-drop format using 24-well VDX plates (Hampton Research). In this case, the well volume was increased to 1 ml.
Prior to cryogenic data collection, crystals were given a brief soak (<30 s) in cryoprotectant, which corresponded to the crystallization solution supplemented with 20%(v/v) ethylene glycol in place of an equivalent volume of buffer. Crystals were routinely transferred from one solution to another and ultimately mounted for X-ray data collection using cryo-loops (Hampton Research). Crystals were flash-cooled by plunging into liquid nitrogen and stored prior to transport to the synchrotron. For data collection, a single crystal was transferred to the goniostat on station PX14.1 at the Daresbury Synchrotron Radiation Source using Hampton Research cryotools and maintained at 100 K with a Cryojet cryocooler (Oxford Instruments). Diffraction data were recorded on a Quantum 4 CCD detector (Area Detector Systems Corp.) using an X-ray wavelength of 1.488 Å. The diffraction data were integrated using MOSFLM (Leslie, 2006 ▶) and scaled using SCALA (Evans, 2006 ▶). Further analysis of the data was performed using programs from the CCP4 suite (Collaborative Computational Project, Number 4, 1994 ▶).
3. Results and discussion
AbsC was overexpressed and purified with a final yield of approximately 10 mg of protein from 1 l culture and was judged to be greater than 98% pure by SDS–PAGE analysis. The DLS analysis after gel filtration showed a monomodal distribution, with a polydispersity value of 18.9% and a molecular-size estimate of 39.6 kDa. This was comparable to the value of 36 kDa estimated from the calibrated gel-filtration column, strongly suggesting that AbsC exists as a homodimer in solution (calculated molecular weight of 41.0 kDa for the His-tagged dimer), in agreement with other characterized MarR homologues.
Preliminary crystals grew within 24 h at 293 K from several different crystallization conditions. Improved crystals were subsequently obtained with a precipitant solution consisting of 1 M ammonium sulfate, 0.55 M potassium sodium tartrate in 100 mM citrate pH 5.6, giving crystals with approximate dimensions of 700 × 50 × 50 µm (Fig. 1 ▶).
Figure 1.
Single crystal of S. coelicolor AbsC of approximately 700 × 50 × 50 µm in size.
Native X-ray data were collected from a single AbsC crystal: a total of 214 × 0.4° oscillation images were recorded in a continuous sweep to a maximum resolution of 2.25 Å. Indexing was consistent with a primitive orthorhombic lattice, with unit-cell parameters a = 43.53, b = 121.30, c = 143.75 Å. After processing the data in space group P222, pseudo-precession plots were analysed using HKLVIEW (Collaborative Computational Project, Number 4, 1994 ▶) and the systematic absences were indicative of space group P212121. Reprocessing in this space group yielded a data set that was 95.6% complete to 2.25 Å resolution. Data-collection statistics are summarized in Table 1 ▶.
Table 1. Summary of X-ray data for AbsC.
Values in parentheses are for the outer resolution shell.
| Wavelength (Å) | 1.488 |
| Resolution range (Å) | 32.22–2.25 (2.37–2.25) |
| Unique reflections | 35348 (3925) |
| Completeness (%) | 95.6 (75.9) |
| Redundancy | 3.1 (2.2) |
| Rmerge† | 0.045 (0.153) |
| 〈I/σ(I)〉 | 18.2 (6.4) |
| Wilson B value (Å2) | 38.1 |
R
merge = 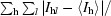
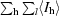 , where I
hl is the lth observation of reflection h and 〈I
h〉 is the weighted average intensity for all observations l of reflection h.
, where I
hl is the lth observation of reflection h and 〈I
h〉 is the weighted average intensity for all observations l of reflection h.
Estimation of the content of the asymmetric unit (ASU) suggested that three or four His-tagged AbsC subunits were most likely, giving solvent contents of 60.3% or 47.0% and crystal-packing parameters (V M) of 3.09 or 2.32 Å3 Da−1, respectively. Given that both DLS and gel filtration suggest that AbsC is dimeric and that MarR homologues exist as homodimers (Wilkinson & Grove, 2006 ▶), an even number of subunits per ASU, i.e. four, would seem to be the most probable (Matthews, 1968 ▶).
A Patterson function calculated on data in the resolution range 10.0–3.0 Å revealed a significant peak (at 24% of the origin peak) with a vector of u = 0.0, v = 0.5, w = 0.014, indicating that one or more molecules in the ASU were similarly orientated. A self-rotation function calculated on data in the resolution range 10.0–5.0 Å using MOLREP (Vagin & Teplyakov, 2000 ▶) did not show any clear noncrystallographic symmetry (NCS), perhaps suggesting that if NCS axes were present they were parallel (or almost parallel) to crystallographic axes.
Fold prediction, based on the amino-acid sequence of AbsC, was performed using the FUGUE server (http://www-cryst.bioc.cam.ac.uk/~fugue/prfsearch.html; Shi et al., 2001 ▶). This found eight ‘certain’ hits, with the top three being MarR (PDB code 1jgs; Z score 21.4; 18% sequence identity), SlyA (PDB code 1lj9; Z score 18.4; 16% sequence identity) and YusO (PDB code 1s3j; Z score 15.7; 16% sequence identity). All three hits were used as search models for molecular replacement in the program AMoRe (Navaza, 1994 ▶), but none yielded plausible solutions against the 2.25 Å resolution native data set, although this is not too surprising given their very low sequence identities. Thus, we will need to solve the AbsC structure by isomorphous replacement methods. To this end, we are preparing selenomethionine-labelled protein; there are a total of seven methionine residues present in the 158-amino-acid sequence of AbsC.
Acknowledgments
This work was funded by the BBSRC. HK was supported by a research fellowship from the Deutsche Forschungsgemeinschaft (KO 2901/1-1). We would like to thank A. Page for help with overexpression and S. Keller, I. Gómez García and the station manager of PX14.1, M. MacDonald, for assistance with data collection.
References
- Alekshun, M. N., Levy, S. B., Mealy, T. R., Seaton, B. A. & Head, J. F. (2001). Nature Struct. Biol.8, 710–714. [DOI] [PubMed] [Google Scholar]
- Camilli, A. & Bassler, B. L. (2006). Science, 311, 1113–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collaborative Computational Project, Number 4 (1994). Acta Cryst. D50, 760–763. [Google Scholar]
- Evans, P. (2006). Acta Cryst. D62, 72–82. [DOI] [PubMed] [Google Scholar]
- Hong, M., Fuangthong, M., Helmann, J. D. & Brennan, R. G. (2005). Mol. Cell, 20, 131–141. [DOI] [PubMed] [Google Scholar]
- Leslie, A. G. W. (2006). Acta Cryst. D62, 48–57. [DOI] [PubMed] [Google Scholar]
- Matthews, B. W. (1968). J. Mol. Biol.33, 491–497. [DOI] [PubMed] [Google Scholar]
- Miller, P. F. & Sulavik, M. C. (1996). Mol. Microbiol.21, 441–448. [DOI] [PubMed] [Google Scholar]
- Navaza, J. (1994). Acta Cryst. A50, 157–163. [Google Scholar]
- Rouanet, C., Reverchon, S., Rodionov, D. A. & Nasser, W. (2004). J. Biol. Chem.279, 30158–30167. [DOI] [PubMed] [Google Scholar]
- Shi, J., Blundell, T. L. & Mizuguchi, K. (2001). J. Mol. Biol.310, 243–257. [DOI] [PubMed] [Google Scholar]
- Spory, A., Bosserhoff, A., von Rhein, C., Goebel, W. & Ludwig, A. (2002). J. Bacteriol.184, 3549–3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier, F. W. & Moffatt, B. A. (1986). J. Mol. Biol.189, 113–130. [DOI] [PubMed] [Google Scholar]
- Vagin, A. & Teplyakov, A. (2000). Acta Cryst. D56, 1622–1624. [DOI] [PubMed] [Google Scholar]
- Wilkinson, S. P. & Grove, A. (2004). J. Biol. Chem.279, 51442–51450. [DOI] [PubMed] [Google Scholar]
- Wilkinson, S. P. & Grove, A. (2006). Curr. Issues Mol. Biol.8, 51–62. [PubMed] [Google Scholar]
- Wyborn, N. R., Stapleton, M. R., Norte, V. A., Roberts, R. E., Grafton, J. & Green, J. (2004). J. Bacteriol.186, 1620–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]



