Figure 4. Xenopus Rab32 is an A-Kinase Anchoring Protein.
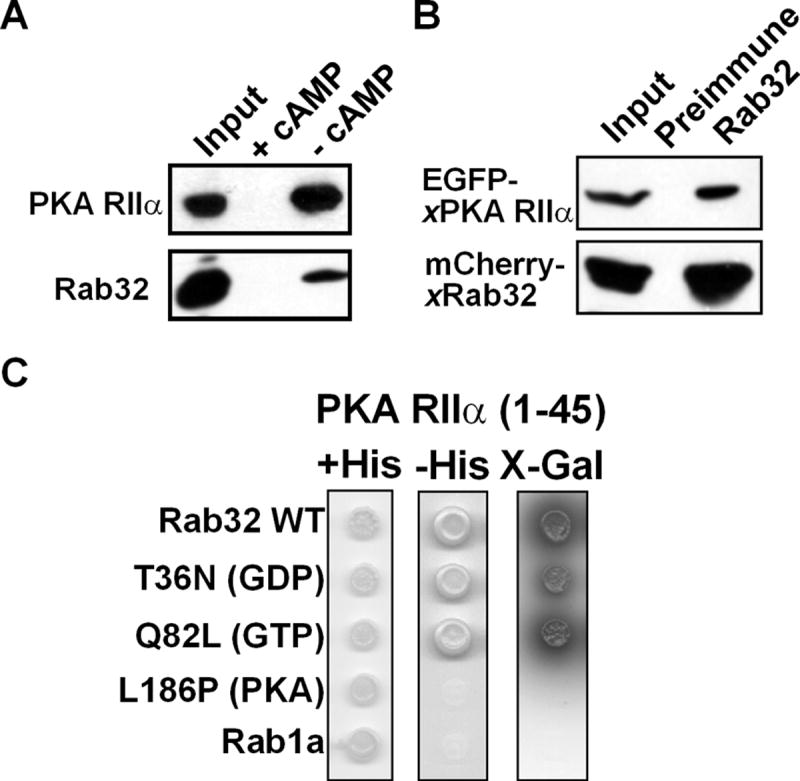
(A) PKA RIIα binds Xenopus Rab32. Xenopus melanophore extracts were incubated with cAMP-agarose resin in the presence of 75 mM cAMP (+ cAMP) or in the absence cAMP (- cAMP). cAMP-agarose resin was washed and eluted with 75mM cAMP. The Western blot was performed using PKA RIIα and xRab32 antibodies. The cAMP agarose resin binds RIIα and also pulls down xRab32. This interaction is abolished in the presence of 75 mM cAMP.
(B) Rab32 binds to PKA RIIα in vivo. Extracts from cells coexpressing EGFP-xPKA RIIα and mCherry-xRab32 were immunoprecipitated with an anti-Rab32 antibody or preimmune IgG. Precipitates were probed using anti-PKA RIIα antibody or anti-Rab32 and developed using HRP-protein A. Note that Rab32 antibody but not the preimmune IgG pulls down PKA RIIα. Inputs are 5% of cell extracts from sample.
(C) Two-hybrid analysis of Rab32-RIIα binding. xRab32 bait constructs (lacking C-terminal cysteins to prevent membrane binding) were tested against the indicated prey constructs in the yeast two-hybrid system for the ability to grow on minimal media in the presence (+His), or absence (-His) of histidine. The Rab32- PKA RIIα interaction was also tested with high-stringency (SD/-Trp/-Leu/-His/-Ade/X-α-Gal) plates. Growth on minimal media in the absence of histidine or α-galactosidase activity represents a positive interaction.
