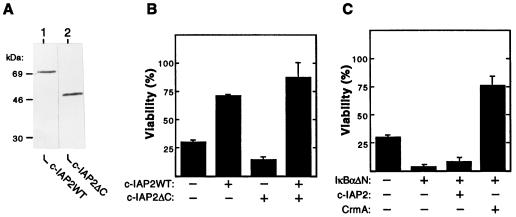
Figure 3.
Human c-IAP2 protects cells from TNF-induced killing via an NF-κB-dependent mechanism. (A) HeLa cells were transfected with 10 μg of the indicated FLAG-tagged expression vectors. Cytoplasmic extracts were prepared 48 hr after transfection and fractionated by SDS/PAGE. Proteins were transferred to polyvinylidene difluoride membranes and subjected to immunoblotting with monoclonal anti-FLAG antibodies. (B) Effects of wild-type and mutant c-IAP2 on TNF-induced death. HeLa cells (3 × 105) were cotransfected with pZeoSVLacZ DNA (1 μg) and pCMV4-based expression plasmids (1 μg) encoding either c-IAP2WT or c-IAP2ΔC. Total DNA input was normalized with pCMV4. After 24 hr of growth, transfected cultures were split into 24-well plates and propagated for 24 hr in the presence or absence of TNF (15 ng/ml). Cells were stained for β-galactosidase expression and scored. Cell viability is expressed as the percentage of blue cells in TNF-treated versus unstimulated cultures. The data shown represent the mean viability (± SEM) of cells determined from triplicate transfections in two separate experiments. (C) Requirement of NF-κB for c-IAP2-mediated protection. HeLa cells were cotransfected with pZeoSVLacZ (1 μg) and the indicated combinations of expression vectors for IκBαΔN (0.1 μg), c-IAP2 (1 μg), and CrmA (1 μg). The percentage of cells (± SEM) surviving exposure to TNF (24 hr) under each experimental condition was determined as described for B. Similar results were obtained in three separate experiments.