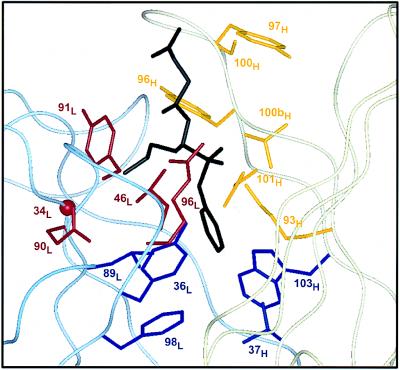
Figure 3.
Design of hu17E8 libraries. Residues targeted for randomization are shown on the structure of 17E8 bound to the norleucine phosphonate hapten (19). The antibody light chain is represented by a pale blue ribbon, the heavy chain in pale yellow and bound hapten is black. Library 1 (red) targeted residues that help form the binding pocket of the substrate side chain; Gly-34L (CDR-L1), Leu-46L (FR-L2), Gln-90L, Tyr-91L, Arg-96L (CDR-L3). Library 2 (yellow) randomized heavy chain residues in CDR-H3 and also included a binary randomization of Lys-93H; Lys-93H (FR-H3), Tyr-96H, Tyr-97H, Ser-100H, Val-100bH, Asp-101H (CDR-H3). Library 3 (blue) randomized hydrophobic residues at the bottom of the substrate/hapten binding pocket; Tyr-36L (FR-L2), Leu-89L (CDR-L3), Phe-98L (FR-L4), Val-37H (FR-H2), Trp-103H (FR-H4). Omitted from these libraries were residues His-35H and Ser-95H; given their putative role in the catalytic mechanism of 17E8 (19), we chose not to randomize these positions. FR, framework region. All residues are numbered according to Kabat et al. (23).