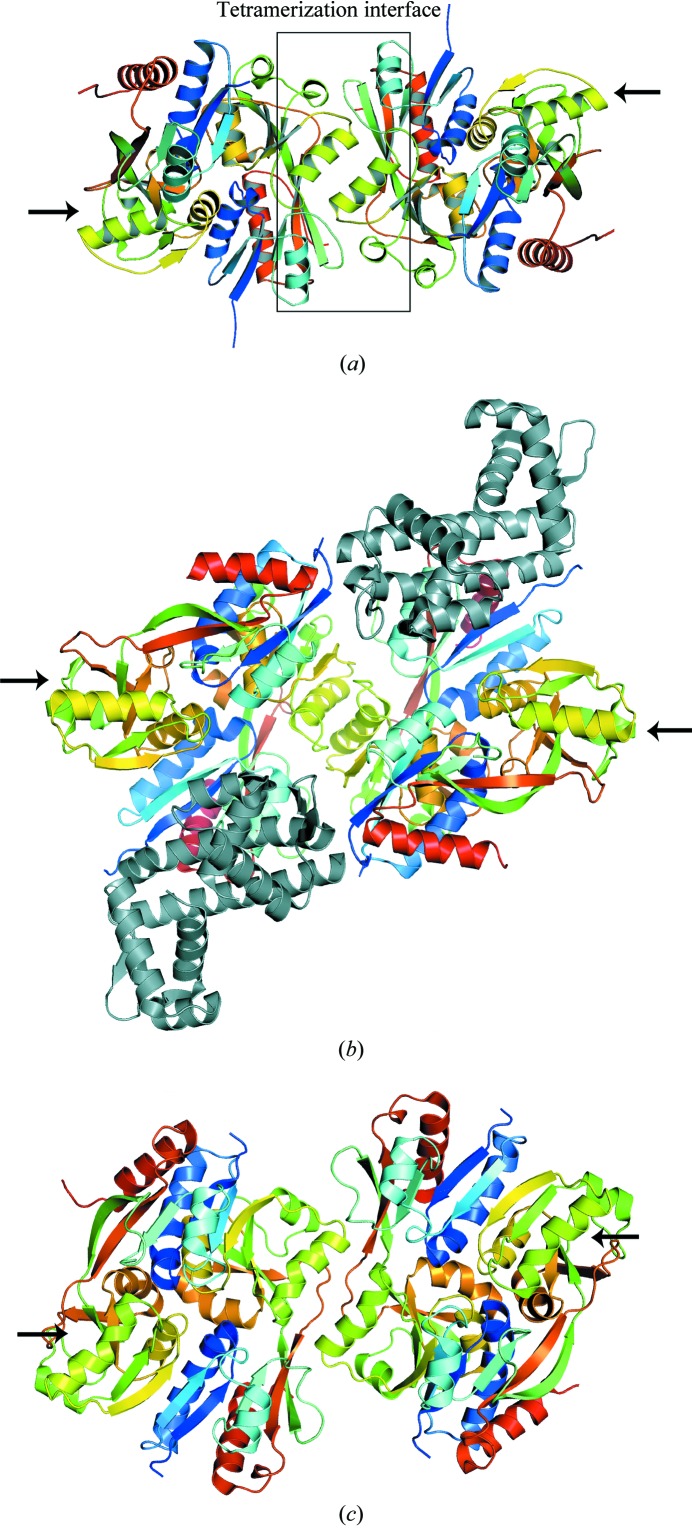
Figure 2.
Ribbon representations of the BenM EBD tetramer structure A in the P4322 space group (a) and the full-length LTTRs CbnR (b) and DntR (c). The secondary structures of each subunit are colored from blue to red going from the N-terminus of the effector-binding domain to the C-terminus. Arrows point to unliganded tetramerization sites. The tetramerization interface of BenM is shown within a box. The yellow–green helices centered within the box are BenM EBD helix αH6. The structurally homologous helices in CbnR and DntR are also colored yellow–green. The DNA-binding domains of CbnR are colored gray. Although DntR crystallized as a full-length protein, no coordinates are available for the DNA-binding domains. The unliganded tetramerization sites are not sterically hindered from forming additional oligomers. We propose that these available sites promote high-order oligomerization and may serve as the basis for the low solubility of this class of proteins under some conditions.