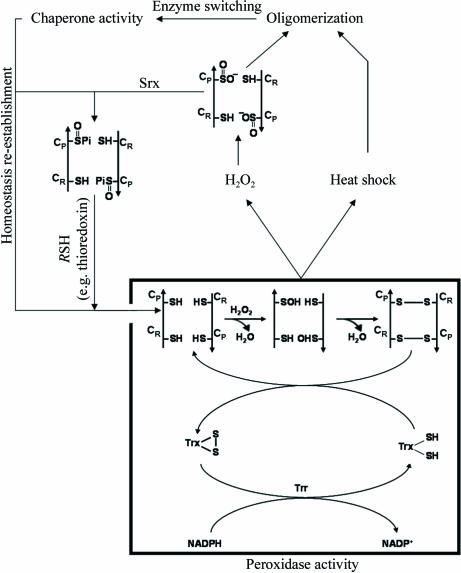
Figure 1.
Molecular switching of thioredoxin peroxidases. At low H2O2 concentrations, CysP (Cys47 in yeast Tsa1) is oxidized to CysP-SOH, which reacts with the resolving cysteine (CysR; Cys170 in Tsa1) of the other subunit in the homodimer to form an intermolecular disulfide. Under physiological conditions, the enzyme is reduced by the thioredoxin system (represented by Trx, Trr and NADPH). The peroxidase inactivation is the result of heat shock or oxidative stress. In the case of oxidative stress the overoxidation occurs at CysP (Cys47 in yeast Tsa1), resulting in CysP-SO2H. After re-establishment of homeostasis, peroxidase activity is restored by reduction of the CysP-SO2H moiety in a reaction that requires ATP hydrolysis and is catalyzed by Srx, with reducing equivalents being provided by physiological thiols (RSH) such as Trx.