Abstract
Background
Recent years have seen the development of various pathway-based methods for the analysis of microarray gene expression data. These approaches have the potential to bring biological insights into microarray studies. A variety of methods have been proposed to construct networks using gene expression data. Because individual pathways do not act in isolation, it is important to understand how different pathways coordinate to perform cellular functions. However, there are no published methods describing how to build pathway clusters that are closely related to traits of interest.
Results
We propose to build pathway clusters from pathway-based classification methods. The proposed methods allow researchers to identify clusters of pathways sharing similar functions. These pathways may or may not share genes. As an illustration, our approach is applied to three human breast cancer microarray data sets. We found that our methods yielded consistent and interpretable results for these three data sets. We further investigated one of the pathway clusters found using PubMatrix. We found that informative genes in the pathway clusters do have more publications with keywords, like estrogen receptor, compared with informative genes in other top pathways. In addition, using the shortest path analysis in GeneGo's MetaCore and Human Protein Reference Database, we were able to identify the links which connect the pathways without shared genes within the pathway cluster.
Conclusion
Our proposed pathway clustering methods allow bioinformaticians and biologists to investigate how informative genes within pathways are related to each other and understand possible crosstalk between pathways in a cluster. Therefore, building pathway clusters may lead to a better understanding of molecular mechanisms affecting a trait of interest, and help generate further biological hypotheses from gene expression data.
Background
The increasing use of high-throughput microarray technologies in biological and biomedical research has motivated many novel statistical and computational approaches to analyze such data. They can be applied to (1) identify differentially expressed genes, (2) discover subclasses through clustering, and (3) classify subjects into known classes. Although most of these methods either examine one gene at a time, i.e. single-gene based, or all the genes at the same time, a number of methods investigate a set of genes at a time, where the gene-set information can come from various external databases, such as KEGG [1], BioCarta [2] and GenMapp [3]. These curated gene-sets or pathways from biological experiments often serve a particular cellular or physiological function. These gene-set based (or pathway-based) methods include Gene Set Enrichment Analysis (GSEA) [4], Random Forests [5], Hotelling's T2 [6], and Significance Analysis of Microarray to gene-set analyses (SAM-GS) [7]. Although it is unlikely that one particular method will be superior to others for all the data sets, these methods seem to be able to generate biologically meaningful results for different data sets. In addition, pathway-based tests can identify more subtle changes in expression than single gene based tests [8]. Furthermore, pathway-based methods can generate biological hypotheses more effectively based on prior knowledge. These hypotheses may be readily tested using complementary approaches, e.g. proteomics and metabolomics analyses.
It is well known that different pathways do not work in isolation. In fact, each pathway is part of an overall biological network. Therefore, it is natural to ask how different pathways, or gene-sets, coordinate their activities. In the context of using gene expression data to predict a trait of interest, e.g. cancer, some pathways may function in a coherent fashion whereas others may have independent functions or effects on phenotypes. Despite the importance of this topic, there is scant literature on relating different pathways. In this paper, we propose to cluster pathways that have similar effects on the phenotype of interest. Our approach is built on our previous proposal of adopting the Random Forests approach for pathway analysis [5]. The Random Forests approach has been found to perform very well among a number of machine learning methods in pathway-based classification. To extend the Random Forests approach for pathway cluster analysis, we use class votes from Random Forests to build pathway clusters related to phenotype of interest. As detailed below in the Methods section, class votes can provide a measure of the similarity between two subjects' gene expression profiles for a given pathway. This measure can then be used to define similarities, or distances, between pathways. Based on these inferred pathway distances, we then use the Tight Clustering [9] approach to identify pathway clusters. The identification of such clusters may provide useful information for biologists to generate hypotheses on the underlying disease mechanisms. Pathway clusters may also help identify novel biomarkers for screening or serving as drug targets for combination therapy.
The rest of the paper is organized as follows. The detailed methodology is discussed in the Methods section. In the Results section, we demonstrate the usefulness of this approach through the application of our methods to three different breast cancer microarray data sets to uncover pathway clusters that are involved in estrogen receptor (ER) status classification. We conclude the paper in the Discussion and Conclusions sections.
Methods
We first briefly review the Random Forests approach for pathway analysis [5]. Random Forests constructs many classification trees and thus the name 'forest'. For each pathway, the input data for Random Forest would be a gene expression matrix of the genes belonging to the pathway by the number of subjects in the data set. Every tree in a Random Forests is built using a deterministic algorithm and the trees are different from the ordinary tree algorithms (e.g. CART) owing to two factors. First, at each node, a best split is chosen from a random subset of the predictors rather than all of them. Second, every tree is built using a bootstrap sample of the original observations. A subject is put down a tree for classification using the input vector of gene expression for genes within a particular pathway. The tree gives a classification and decides which class this subject belongs to. In the end, the forests choose the class that gives the majority votes for each subject. The out-of-bag (OOB) data, approximately one-third of the observations, are then used to estimate the prediction accuracy. Small classification error based on genes in a given pathway would indicate the pathway as potentially interesting [5].
We can build for each pathway a Random Forest to predict an individual's phenotype based on his/her gene expression levels within this pathway. To define whether two pathways have similar effects on an individual's phenotype, we can use the pathway prediction results to define their similarities. For example, if two pathways always give the same phenotype prediction based on gene expression data in these pathways, we infer that these two pathways have similar functions. To realize this idea, we use an output from Random Forests, class votes, to define pathway distances that can be used to build pathway clusters.
Class Votes
To define class votes, for each study subject, the proportion of votes for a specific class is recorded based on the prediction results from individual trees in the Random Forest. Therefore, every pathway defines a class vote matrix of length n by k, where n is the number of samples in the study and k is the number of classes. In case of only two classes, we can use the votes of one class to represent the confidence of each individual belonging to that particular class. For example, for subject A, if we have 0.15 for class 1 and 0.85 for class 2, that means subject A has been voted to be class 2 85% of the time. Therefore, two pathways can be thought to have similar effects on the phenotype if the class vote matrices/vectors from these two pathways are similar.
Building Pathway Clusters
Based on class votes, we propose to use Tight Clustering to infer pathway clusters.
Tight Clustering is a robust method using re-sampling for clustering and pattern recognition [9]. It finds tight and stable clusters in a sequential manner. The K-means algorithm is applied iteratively, along with the calculations of the average co-membership matrices and similarity measures of cluster sets. When performing Tight Clustering on the class votes for a pair of pathways, the Euclidean distance between them is used. Tight Clustering does not explicitly estimate the number of clusters, but allows the user to specify the target number of Tight Clusters. It is usually infeasible to estimate the number of clusters since it is not uncommon to see figures that give clear and informative pattern for different number of clusters. For more details of the algorithm, see [9]. As mentioned in their paper, because microarray analysis is an exploratory tool to guide further biological investigations which could potentially be costly, some genes, called scattered genes in their paper, should be left out of the Tight Clusters. This is also true in the pathway-based context. Tight Clusters of class votes for pathways are found and pathways that are not related to other pathways should be left without being clustered. We varied the target number of Tight Clusters from 5 to 20 in analyzing the breast cancer data sets. Tight Clusters which contain two or more top ranked pathways with low OOB error rates are investigated further and pathway clusters are built from them. A heatmap can be used to visualize the Tight Clustering output.
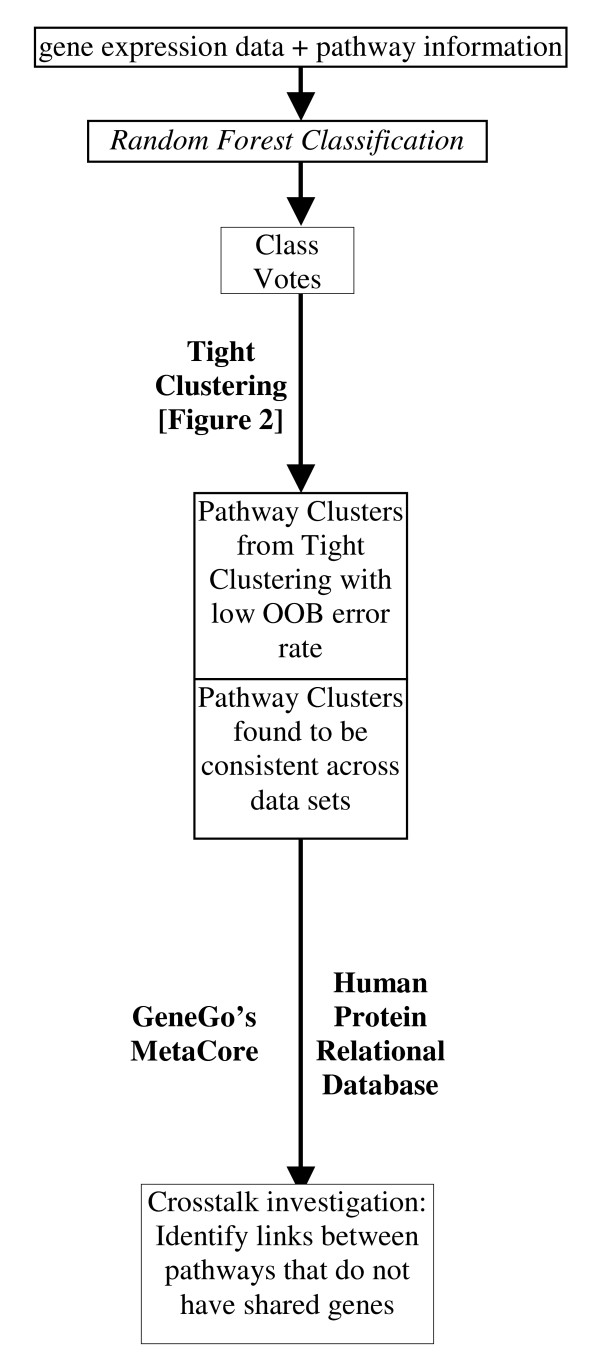
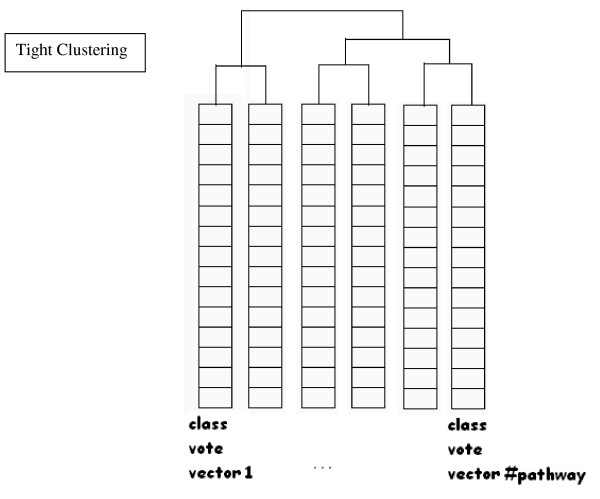
Schematic diagrams of our proposed methods are given in Figures 1 and 2.
Figure 1.
A Schematic Diagram of How to Identify Clusters of Pathways. Pathway (gene sets) information from externally available database, such as KEGG, BioCarta and GenMapp is combined with gene expression from clinical studies. We perform pathway-based Random Forests classification to obtain Class Votes. We identify clusters of pathways containing pathways with low OOB error rate using Tight Clustering. We identify the clusters of pathways that are consistent among different data sets. These pathway clusters are investigated further for possible crosstalk among them.
Figure 2.
Tight Clustering. A diagram illustrating Tight Clustering on Class votes.
Data sets
Pathways
A total of 495 pathways were used for the analysis. These pathways are wired diagrams of a set of predefined genes and molecules from KEGG [1], BioCarta [2], and GenMapp [3] databases. Every pathway in these databases contains a set of genes that are related to some cellular, molecular and/or physiological functions from earlier experiments. These genes are then mapped to the corresponding probes IDs on the microarray chipsets. The distribution is as follows:
(1) A total of 151 pathways were taken from KEGG, a pathway database with the majority responsible for metabolism, degradation and biosynthesis. There are also a few signal or information processing pathways and others related to human diseases and drug development.
(2) We considered 283 BioCarta pathways. Most of these pathways are related to signal transduction for human with a smaller group of metabolic pathways.
(3) The 61 GenMapp pathways we used consist of more genes per set on average. There are different types of pathways such as metabolic pathways, signal transduction pathways, gene families and subcellular components.
Microarray data
Three different breast cancer microarray data sets were used. All of these studies used Affymetrix GeneChip®, but they are of different versions. Consort data set was based on hgu-133 plus 2.0 with 54,613 probesets whereas the other two, LymphNode and p53 data sets [10,11], were based on an older chip called hgu-133a with 22,215 probesets. Consort [12] data set consists of 99 breast tissue samples with clinical status of estrogen receptor. LymphNode data set consists of frozen tumor samples of 286 lymph-node negative patients who had not received adjuvant systematic treatment [10]. p53 data set is a set of 251 frozen tissues that were sequenced for p53 [11].
We chose the breast cancer data sets and ER positive/negative status (ER+/ER-) to study because breast cancer has been extensively studied in the literature and tumor samples are normally classified on the basis of ER status [13]. A recent publication described a set of prognostic gene expression classifiers for ER+ breast cancer [14]. The estrogen receptor status has also been used to predict breast cancer therapy, breast cancer survival rate and estimate the risk of breast cancer [15-18]. ER+ breast cancers are usually treated with hormone therapy whereas ER- breast cancers are treated using chemotherapy. Not all breast carcinomas are responsive to the treatment though. Thus, there is an urgent need to identify novel therapeutic targets and develop new agents. Moreover, pathway crosstalk and new biological insights might help find predictive biomarkers [19].
To deal with the issue of unbalanced sample size between the ER+ and ER- groups we utilized weighted Random Forests. The p53 data set is the most unbalanced among the three breast cancer data sets we analyzed, it has 213 in the ER+ and 38 in the ER- groups. For more details on why we chose this approach, see the discussion in the see Additional file 1, DMS1.
The above data sets are available for download from the GEO website under the accessions GSE2109, GSE3494 and GSE2034 for the Consort, p53 and LymphNode data sets, respectively. See Table 1.
Table 1.
Breast cancer data sets used in this study
| Data sets | Reference | n | Genes | Response type |
| Consort | INTEGEN | 99 | 54613 | ER status |
| LymphNode | [10] Wang (2005) | 286 | 22215 | ER status |
| p53 | [11] Miller (2005) | 251 | 22215 | ER status |
Software
The library package randomForest v4.5-18 from the R program was used in our analysis [20] for the Balanced Random Forests solution. A modified version of the original Fortran code was used to perform the Weighted Random Forests in our pathway-based context [21]. For pathway clusters visualization, Cytoscape [22] was used.
Biological Significance
We considered using GO terms based enrichment analysis, but Goeman and Bühlmann [23] pointed out that this approach may not be satisfactory and may result in false positives. Therefore, we used two alternative approaches. First, we used PubMatrix [24], a web-based application that identifies genes' citation with keywords of interest. Genes that contribute most in predicting the correct class in pathway-based classification are called informative genes [5]. We compared the informative genes defined by Random Forests classification that were obtained in the pathway cluster sets and examined whether these genes are more likely to have publications with the keywords of interest compared with informative genes from the top pathways not in the pathway cluster. Although importance measure in Random Forests could be biased [25], it is unlikely in our case since we only used normalized gene expression data and did not combine it with other categorical data, such as sequence data described in [25]. Second, we investigated possible pathways crosstalk using GeneGo's MetaCore [26] and Human Protein Reference Database (HPRD) [27]. Shortest path analyses between a pair of genes were performed using GeneGo's MetaCore to assess how close the two genes are related to each other based on the curated database of human protein-protein, protein-DNA and protein compound interactions.
Results
Class Votes
The target number of Tight Clusters, 5, 10, 15 and 20 were chosen and the tuning parameters were as defined in the Tight Clustering manual. We found that the pathway clusters identified when the target number was 5, 10 and 15 were essentially a subset of those in the 20 Tight Clusters case. To facilitate the investigation of pathway crosstalk, we consider a larger number of Tight Clusters, i.e. 20. We considered forming 25, 30, 35 tight clusters in addition to 5, 10, 15, and 20. Most of the clusters discovered in 20 tight clusters run were rediscovered in 25, 30, and 35. Please see Additional files 2 and 3, varysize_5-10-15-20.xls and varysize_20-25-30-35.xls for more details. On page 12 of the manual for the Tight Clustering program, four sets of parameters for tight clusters of size 5, 10, 15 and 20 were suggested. Therefore, we chose 20 tight clusters. Among these 20 inferred Tight Clusters, we selected those clusters containing two or more pathways whose OOB error rates were among the top 22 lowest across all the pathways. Since we aim to pick out the top pathways with the same OOB error rates, if we had chosen the top 20 pathways, we would have missed some pathways with the same OOB error rates. Based on this criterion, the OOB error rates cut off was 15.5%, 15.5%, and 20% for the p53, LymphNode and Consort data sets respectively. In each of the three data sets, three Tight Clusters were selected. These Tight Clusters are listed in Additional file 1, Table A1 for Consort; Table A2 for LymphNode; and Table A3 for p53 data set. A2ii and A3i from Additional file 1 for LymphNode and p53 data sets, respectively, highly resemble each other (Table 2). Apart from the Alzheimer's disease pathway, the other five pathways are overlapped between the LymphNode and p53 data sets. A2iii, A3ii and A1i in the respective data sets are also very similar (Table 3). "Butanoate metabolism", "Propanoate metabolism" and "Valine leucine and isoleucine degradation" appear in each of the three Tight Clusters of the three different data sets. The A3iii Tight Cluster in p53 data set is a subset of a much larger Tight Cluster A2i in LymphNode, see Additional file 1, Tables A2 and A3 for more details.
Table 2.
Tight Cluster Results 1
| LymphNode (A2ii in Additional file 1) | OOB error(%) | Number of probes |
| BC-Pelp1_Modulation_of_Estrogen_Receptor | 15.38 | 20 |
| Alzheimer's_disease | 17.13 | 23 |
| BC-Deregulation_of_CDK5_in_Alzheimers_Disease | 16.08 | 24 |
| BC-Downregulated_of_MTA_3_in_ER_negative_Breast_Tumors | 12.24 | 26 |
| BC-GATA3_participate_in_activating_the_Th2_cytokine | 11.89 | 33 |
| Nitrogen_metabolism | 14.34 | 40 |
| Gene Symbols of informative genes in this pathway cluster | ||
| MAPK3, PELP1, ESR1, PDZK1, HSPB1, CA12, GLS, IL5, JUNB, GATA3, MAP2K3, MAPT, STH, CSNK1A1 | ||
| p53 (A3i in Additional file 1) | ||
| BC-Pelp1_Modulation_of_Estrogen_Receptor | 13.94 | 20 |
| BC-Downregulated_of_MTA_3_in_ER_negative_Breast_Tumors_ | 15.54 | 26 |
| BC-GATA3_participate_in_activating_the_Th2_cytokine | 13.55 | 33 |
| Nitrogen_metabolism | 17.13 | 40 |
| BC-CARM1_and_Regulation_of_the_Estrogen_Receptor | 14.34 | 54 |
| Gene Symbols of informative genes in this pathway cluster | ||
| MAPK3, PELP1, ESR1, PDZK1, HSPB1, HDAC2, CA12, GLS, IL5, JUNB, GATA3, MAP2K3 | ||
The bold pathways are those with low OOB error rates
Table 3.
Tight Cluster Results 2
| LymphNode (A2iii in Additional file 1) | OOB error(%) | Number of probes |
| beta_Alanine_metabolism | 16.08 | 42 |
| Alanine_and_aspartate_metabolism | 16.78 | 42 |
| Glutamate_metabolism | 16.08 | 50 |
| Butanoate_metabolism | 16.08 | 59 |
| Propanoate_metabolism | 17.83 | 59 |
| Valine_leucine_and_isoleucine_degradation | 14.69 | 71 |
| Gene Symbols of informative genes in this pathway cluster | ||
| ABAT, ALDH1A3, GLUL, GMPS, HMGCL, HSD17B4, MAP3K15, MCCC2, PDHA1 | ||
| p53 (A3ii in Additional file 1) | ||
| Alanine_and_aspartate_metabolism | 17.53 | 42 |
| Butanoate_metabolism | 15.54 | 59 |
| Propanoate_metabolism | 18.33 | 59 |
| GM-Glycolysis_and_Gluconeogenesis | 23.11 | 66 |
| Valine_leucine_and_isoleucine_degradation | 15.54 | 71 |
| Gene Symbols of informative genes in this pathway cluster | ||
| ABAT, ALDH1A3, HMGCL, HSD17B4, MAP3K15, MCCC2, PDHA1 | ||
| Consort (A1i in Additional file 1) | ||
| Glycosphingolipid_biosynthesis | 23.23 | 34 |
| BC-GATA3_participate_in_activating_the_Th2_cytokine | 14.14 | 43 |
| Butanoate_metabolism | 15.15 | 82 |
| Propanoate_metabolism | 21.21 | 85 |
| Valine_leucine_and_isoleucine_degradation | 17.17 | 25 |
| Gene Symbols of informative genes in this pathway cluster | ||
| ABAT, GATA3, HSD17B4, MCCC2, PRKAR1B | ||
The bold pathways are those with low OOB error rates
Pathway Clusters
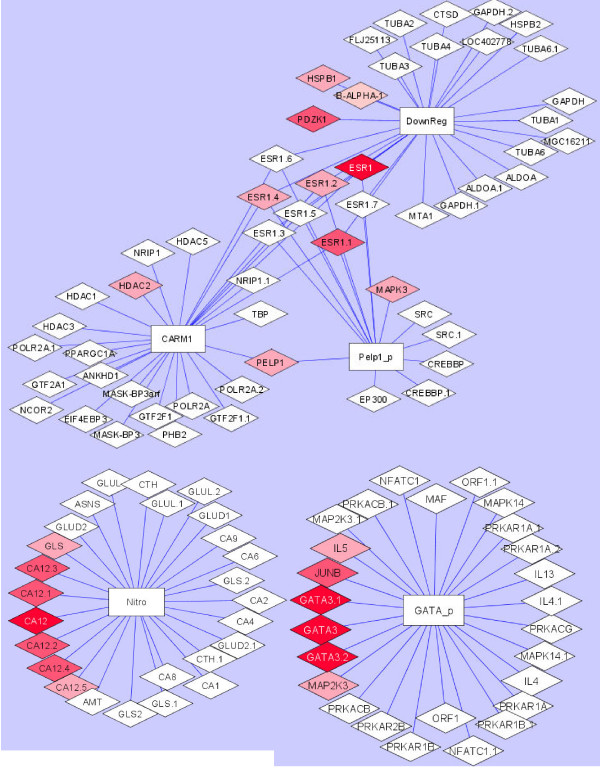
We further investigate the pathway cluster (Table 2) found from the previous section. Figure 3 consists of three pathway clusters built from the overlapped pathways. It can be seen that "GATA3 participates in activating the Th2 cytokine gene pathway" and "Nitrogen Metabolism pathway" do not have any overlapping probes with the other 3 pathways. The ESR1 gene is shared among 3 pathways: "PELP1 Modulation of Estrogen Receptor Activity pathway", "CARM1 and Regulation of the Estrogen Receptor pathway", and "Downregulated of MTA 3 in ER negative Breast Tumors pathway". In addition to ESR1, the PELP1 and CARM1 pathways share the informative PELP1 gene. Genes, such as RARA, PGR, PDZK1, HSPB1, HDAC2, and MAPK3 that are not shared also show some importance in classifying subjects.
Figure 3.
Pathway Clusters. A pathway cluster showing a total of five pathways, three of which have shared genes and two pathways do not share common genes.
PubMatrix
To more systematically study the biological significance of the results, we looked at the publications of the top informative genes (top two genes in each pathway) with keywords, like breast cancer, estrogen receptor, and progesterone receptor, of interest. We examined the top informative genes from the pathway cluster in Table 2, which consists of 5 pathways, with two of them that do not have any overlapping probes with the rest. It is evident from PubMatrix search that the proportions of these informative genes in the pathway cluster do show a higher number of literature support compared with the informative genes outside of the pathway cluster (Table 4). This is true for the informative genes for all three data sets and more so for the p53 data set. To assess the significance of these results, Fisher's Exact Test was performed. For breast cancer citations, the p-values were 0.149, 0.002, and 0.061 for data sets, LymphNode, p53, and Consort, respectively (Table 5). This indicates a significantly higher proportion of citations related to breast cancer for genes in pathway cluster of Table 2 compared to other informative genes in the top pathways for the p53 data set. The result for Consort just misses the significant cutoff of 0.05, and it is not significant for the LymphNode data set. It was not surprising to see more significant results for estrogen receptor citations, since we are specifically doing classification on the ER+/ER- status. All of the p-values are significant; 0.048, 0.0006, and 0.0084 for data sets LymphNode, p53, and Consort, respectively (Table 6).
Table 4.
Proportion of genes showing more than the indicated number of literature support
| Informative genes not in pathway cluster (Table 2) of top 22 pathways for LymphNode | BC | ER | PR |
| ≥ 1 | 0.44 | 0.33 | 0.20 |
| ≥ 2 | 0.31 | 0.27 | 0.18 |
| ≥ 5 | 0.24 | 0.20 | 0.09 |
| LymphNode (pathway cluster) | BC | ER | PR |
| ≥ 1 | 0.42 | 0.42 | 0.33 |
| ≥ 2 | 0.42 | 0.42 | 0.25 |
| ≥ 5 | 0.25 | 0.33 | 0.17 |
| Informative genes not in pathway cluster (Table 2) of top 22 pathways for p53 | BC | ER | PR |
| ≥ 1 | 0.41 | 0.35 | 0.20 |
| ≥ 2 | 0.33 | 0.28 | 0.13 |
| ≥ 5 | 0.24 | 0.24 | 0.11 |
| p53 (pathway cluster) | BC | ER | PR |
| ≥ 1 | 1.00 | 1.00 | 0.75 |
| ≥ 2 | 1.00 | 0.75 | 0.63 |
| ≥ 5 | 0.63 | 0.63 | 0.25 |
| Informative genes not in pathway cluster (Table 2) of top 22 pathways for Consort | BC | ER | PR |
| ≥ 1 | 0.50 | 0.22 | 0.16 |
| ≥ 2 | 0.44 | 0.38 | 0.25 |
| ≥ 5 | 0.34 | 0.28 | 0.13 |
| Consort (pathway cluster) | BC | ER | PR |
| ≥ 1 | 0.88 | 0.75 | 0.63 |
| ≥ 2 | 0.75 | 0.63 | 0.50 |
| ≥ 5 | 0.50 | 0.50 | 0.25 |
BC = breast cancer, ER = estrogen receptor, PR = progesterone receptor
Table 5.
Breast Cancer Citations
| LymphNode | In citation | Not in citation | p-value |
| Genes in pathway cluster (Table 2) | 8 | 4 | |
| Informative genes not in pathway cluster (Table 2) of top 22 pathways | 20 | 25 | 0.149 |
| p53 | In citation | Not in citation | p-value |
| Genes in pathway cluster (Table 2) | 8 | 0 | |
| Informative genes not in pathway cluster (Table 2) of top 22 pathways | 19 | 27 | 0.002 |
| Consort | In citation | Not in citation | p-value |
| Genes in pathway cluster (Table 2) | 7 | 1 | |
| Informative genes not in pathway cluster (Table 2) of top 22 pathways | 16 | 16 | 0.061 |
Table 6.
Estrogen Receptor Citations
| LymphNode | In citation | Not in citation | p-value |
| Genes in pathway cluster (Table 2) | 8 | 4 | |
| Informative genes not in pathway cluster (Table 2) of top 22 pathways | 16 | 30 | 0.048 |
| p53 | In citation | Not in citation | p-value |
| Genes in pathway cluster (Table 2) | 8 | 0 | |
| Informative genes not in pathway cluster (Table 2) of top 22 pathways | 15 | 30 | 0.0006 |
| Consort | In citation | Not in citation | p-value |
| Genes in pathway cluster (Table 2) | 6 | 2 | |
| Informative genes not in pathway cluster (Table 2) of top 22 pathways | 7 | 25 | 0.0084 |
Possible Pathway Crosstalk
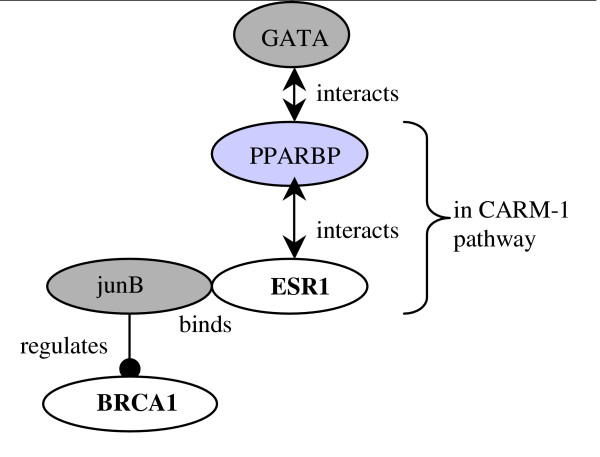
From the previous section, we have seen that even though there are no overlapping genes between both "Nitrogen metabolism" and "GATA3 participate in activating Th2 cytokine genes expression" pathways with other pathways containing ESR1, they appear to form a tight pathway cluster. In order to further understand the possible crosstalk between them, we looked at HPRD and GeneGo's MetaCore. We found connections between GATA3 pathway and CARM-1 pathway from HPRD. This is illustrated in Figure 4, where the dark grey oval genes GATA and junB in GATA3 pathway interacts with PPARBP and ESR1 in CARM-1 pathway. The gene PPARBP, Peroxisome proliferator-activated receptor binding protein, is determined to be at a high level of expression and amplified in breast cancer [28]. In Figure A5 in Additional file 1, it suggests how different proteins receive signals from ESR1 and act upon HIF-1 to regulate CA12.
Figure 4.
Links between GATA3 and CARM1 pathways using HPRD. The connection between genes in GATA3 and CARM1 pathways using information obtained from HPRD.
Shortest Path Analyses
To investigate the possibility of pathway crosstalk further, we searched for the shortest path between GATA3 and CA12 with other top informative genes in the network of all links in the database of GeneGo's MetaCore. This tool assists in finding regulatory paths between two or more genes of interest. The results are shown in Tables 7 and 8. For both GATA3 and CA12, they are the genes with the least number of gene steps to the gene ESR1, with 2 and 3 steps, respectively. It furthers strengthens our belief that the pathways GATA3 and Nitrogen Metabolism are closely tied with the other four pathways within the pathway cluster. The number of links with a distance of two between GATA3 and ESR1 is 6, which is much larger than ESR1 and EGFR or IKBKB both with just one gene connecting between them. The gene, MUC1, connects ESR1 and EGFR. IKK-alpha is the gene which connects ESR1 and IKBKB. These two genes are a subset of the 6 between GATA3 and ESR1. Furthermore, there are 7 literature support of genes MUC1 and HNF3-alpha [29-35] related to breast neoplasm compared to 6 (MUC1) for EGFR and none for IKBKB. In fact, EGFR is one of the genes in the Calcium Signalling pathway which also share genes with the GATA3 pathway. CA12 and ESR1 are also closely tied; CA12 is connected to ESR1 through HIF-1 and NCOA1. There are 4 literature support of genes HIF-1 and NCOA1 related to breast neoplasm [36-39]. Again, the EGFR is at the top of this chart with the same number gene steps but with 4 different paths, and two more literature support than CA12. Another gene IL6ST has two different paths and three literature support. Although, it seems that the connection between CA12 and the four pathways with ESR1 is not as strong, it is still significant relative to the majority of the top informative genes which show 4 or more gene steps.
Table 7.
Shortest Path between GATA3 and other Genes in the Top 22 Pathways (without overlap with GATA3 pathway)
| GATA3 and | Distance (gene steps) | Number of links with the shortest distance* | Genes with literature related to breast cancer* |
| ESR1 (in pathway cluster) | 2 | 6 [MUC1, SMAD8, IKK-alpha, HDAC4, HNF3-alpha, GATA-1] | 7 |
| EGFR | 2 | 1 [MUC1] | 6 (subset of the 7 above) |
| IKBKB | 2 | 1 [IKK-alpha] | 0 |
| IFNAR | 3 | ||
| GFRA1 | 3 | ||
| IGF1 | 3 | ||
| ATP7B | 3 | ||
| VAV3 | 3 | ||
| COX7c | 3 | ||
| B3GNT6 | 3 | ||
| MYCL1 | 3 | ||
| ACACB | 3 | ||
| UQCRH | 3 | ||
| LYN | 3 | ||
| ACTN1 | 3 | ||
| IL6ST | 3 | ||
| STC2 | 4 | ||
| PDXK | 4 | ||
| CFLAR | 4 | ||
| BBOX1 | 4 | ||
| TARS | 5 | ||
| SSH3 | 5 | ||
| NDUFA9 | 6 | ||
| HMGCL | 6 | ||
| ABAT | 6 | ||
| DAZAP2 | Infinity |
*shown only for the shortest distance
Table 8.
Shortest Path between CA12 and other Genes in the Top 22 Pathways (without overlap with Nitrogen Metabolism pathway)
| CA12 and | Distance (gene steps) | Number of links with the shortest distance* | Genes with literature related to breast cancer* |
| ESR1 (in pathway cluster) | 3 | 1 (HIF-1, NCOA1) | 4 |
| EGFR | 3 | 4 (HIF-1 + MAPK3, Beta-catenin, STAT5B, MAPK1) | 6 |
| IL6ST | 3 | 2 (HIF-1 + MAPK1, MPAK3) | 3 |
| COX7c | 4 | ||
| IGF1R | 4 | ||
| YES | 4 | ||
| ACTIN1 | 5 | ||
| PRKX | 5 | ||
| VAV3 | 5 | ||
| ABAT | 6 | ||
| HMGCL | 6 | ||
| SSH3 | 6 | ||
| TARS | 6 | ||
| ADCY9 | 7 | ||
| BBOX1 | 7 | ||
| PDXK | 7 | ||
| UQCRH | 7 | ||
| B3GNT6 | 9 | ||
| DAZAP2 | Infinity |
*shown only for the shortest distance
Discussions
In this article, we have described a Random Forests-based approach to identify clusters of pathways sharing similar functions. Class votes measure similarity at the individual level. Using the three different breast cancer data sets to classify between estrogen receptor positive and negative status, we found that Tight Clustering for class votes yielded consistent and interpretable results. We also considered other means of measuring the similarity of class votes, such as the similarities between class votes solely by Euclidean distances, but their performance was less consistent than the methods described here. Moreover, another output, proximity matrices, for Random Forests was also investigated, but it was found to be highly correlated with the class votes (see Figures A4.). Bioinformaticians and biologists can make use of the proposed methods to discover pathway clusters, find informative genes shared between pathways and identify genes that bridge between pathways within a pathway cluster. This allows researchers to obtain results that are more closely tied to the biological mechanism of diseases and to examine pathway crosstalk.
Due to the unbalanced nature of the data sets in this study, the weighted random forests (WRF) algorithm was used. WRF seems to perform better than the alternative balanced random forests procedure. Although we are looking at ER+ vs. ER- status for the Consort, p53 and LymphNode data sets, it is reasonable to obtain different pathway clusters from them. This is because the patients were from different clinical settings. The Consort data set consists of patients from a consortium of different breast cancer studies, the p53 data set consists of patients whose tissue were sequenced for p53 and the LymphNode data set only has patients with negative lymph node status.
In this article, we have also demonstrated the biological relevance of our approach using PubMatrix. The number of citations for informative genes within the pathway cluster together with keywords, like estrogen receptor, is enriched compared to other informative genes of top pathways. We have illustrated the use of GeneGo and HPRD to help us understand possible crosstalk among pathway clusters. The shortest path analyses of GATA3 and CA12 show that the informative genes in pathway clusters are closer in terms of regulatory paths than those informative genes in other top pathways. Furthermore, with the aid of a network visualization tool, biologists can investigate how the informative genes are related to each other within the pathway clusters.
Conclusion
The novel methods presented in this article were able to identify pathway clusters related to outcome of interests that are biologically meaningful. Understanding how the informative genes relate and talk with each other within pathway clusters can help generate further biological hypotheses for follow-up studies. These may be tested using other "omics" technologies, such as proteomics and metabolomics. When the outcome variable is continuous, we can employ the Random Forests Regression approach [5] and easily extend what we have described in this article to the regression setting by using the predicted values from the Random Forests output.
In this paper, we have proposed one way to building pathway clusters. It might be possible to utilize output from other pathway-based methods, such as GSEA to determine the similarity in enrichment scores between two pathways and build a graph of pathway network from the calculated similarity measures. Moreover, our approach would encourage other researchers to look into new ways in building pathway clusters and bring fresh insights into microarray analysis.
Authors' contributions
HP and HZ developed this new method for building pathway clusters. HP did the programming and carried out the computational work. Both authors read and approved the manuscript.
Supplementary Material
This file contains supporting information for this paper. These include: a study of weighted random forests vs. balanced random forests, GeneGo MetaCore output and tight clusters and absolute differences results.
Tight Clustering results for 5, 10, 15 and 20 tight clusters.
Tight Clustering results for 20, 25, 30 and 35 tight clusters.
Acknowledgments
Acknowledgements
Support provided in part by National Institute of Health (NIH) grants U24 NS051869 and R01 GM59507. We thank the International Genomics Consortium (IGC) and Expression Project For Oncology (expO) for making one of the data sets available to us. We thank three anonymous reviewers for their valuable comments and suggestions. We also thank Keck Biostatistics Resource at Yale for the GeneGo's MetaCore account and Matthew Holford for providing the informatics support and access to the gene interaction database.
Contributor Information
Herbert Pang, Email: herbert.pang@yale.edu.
Hongyu Zhao, Email: hongyu.zhao@yale.edu.
References
- Kanehisa M, Goto S, Kawashima S, Okuno Y, Hattori M. The KEGG resource for deciphering the genome. Nucleic Acids Res. 2004;32:D277–80. doi: 10.1093/nar/gkh063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BioCarta http://www.biocarta.com/
- Dahlquist KD, Salomonis N, Vranizan K, Lawlor SC, Conklin BR. GenMAPP, a new tool for viewing and analyzing microarray data on biological pathways. Nat Genet. 2002;31:19–20. doi: 10.1038/ng0502-19. [DOI] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang H, Lin A, Holford M, Enerson BE, Lu B, Lawton MP, Floyd E, Zhao H. Pathway analysis using random forests classification and regression. Bioinformatics. 2006;22:2028–2036. doi: 10.1093/bioinformatics/btl344. [DOI] [PubMed] [Google Scholar]
- Kong SW, Pu WT, Park PJ. A multivariate approach for integrating genome-wide expression data and biological knowledge. Bioinformatics. 2006;22:2373–2380. doi: 10.1093/bioinformatics/btl401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinu I, Potter JD, Mueller T, Liu Q, Adewale AJ, Jhangri GS, Einecke G, Famulski KS, Halloran P, Yasui Y. Improving gene set analysis of microarray data by SAM-GS. BMC Bioinformatics. 2007;8:242. doi: 10.1186/1471-2105-8-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstrale M, Laurila E, Houstis N, Daly MJ, Patterson N, Mesirov JP, Golub TR, Tamayo P, Spiegelman B, Lander ES, Hirschhorn JN, Altshuler D, Groop LC. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34:267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- Tseng GC, Wong WH. Tight clustering: A resampling-based approach for identifying stable and tight patterns in data. Biometrics. 2005;61:10–16. doi: 10.1111/j.0006-341X.2005.031032.x. [DOI] [PubMed] [Google Scholar]
- Wang Y, Klijn JG, Zhang Y, Sieuwerts AM, Look MP, Yang F, Talantov D, Timmermans M, Meijer-van Gelder ME, Yu J, Jatkoe T, Berns EM, Atkins D, Foekens JA. Gene-expression profiles to predict distant metastasis of lymphnode-negative primary breast cancer. Lancet. 2005;365:671–679. doi: 10.1016/S0140-6736(05)17947-1. [DOI] [PubMed] [Google Scholar]
- Miller LD, Smeds J, George J, Vega VB, Vergara L, Ploner A, Pawitan Y, Hall P, Klaar S, Liu ET, Bergh J. An expression signature for p53 status in human breast cancer predicts mutation status, transcriptional effects, and patient survival. Proc Natl Acad Sci. 2005;102:13550–13555. doi: 10.1073/pnas.0506230102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Genomics Consortium http://www.intgen.org/
- West M, Blanchette C, Dressman H, Huang E, Ishida S, Spang R, Zuzan H, Olson JA, Marks JR, Nevins JR. Predicting the clinical status of human breast cancer by using gene expression profiles. Proc Natl Acad Sci. 2001;98:11462–11467. doi: 10.1073/pnas.201162998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teschendorff AE, Naderi A, Barbosa-Morais NL, Pinder SE, Ellis IO, Aparicio S, Brenton JD, Caldas C. A consensus prognostic gene expression classifier for ER positive breast cancer. Genome Biol. 2006;7:R101. doi: 10.1186/gb-2006-7-10-r101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang E, Cheng SH, Dressman H, Pittman J, Tsou MH, Horng CF, Bild A, Iversen ES, Liao M, Chen CM, West M, Nevins JR, Huang AT. Gene expression predictors of breast cancer outcomes. Lancet. 2003;361:1590–1596. doi: 10.1016/S0140-6736(03)13308-9. [DOI] [PubMed] [Google Scholar]
- Colditz GA, Rosner BA, Chen WY, Holmes MD, Hankinson SE. Risk factors for breast cancer according to estrogen and progesterone receptor status. J Natl Cancer Inst. 2004;96:218–228. doi: 10.1093/jnci/djh025. [DOI] [PubMed] [Google Scholar]
- Berry DA, Cirrincione C, Henderson IC, Citron ML, Budman DR, Goldstein LJ, Martino S, Perez EA, Muss HB, Norton L, Hudis C, Winer EP. Estrogen-receptor status and outcomes of modern chemotherapy for patients with node-positive breast cancer. JAMA. 2006;295:1658–1667. doi: 10.1001/jama.295.14.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naderi A, Teschendorff AE, Barbosa-Morais NL, Pinder SE, Green AR, Powe DG, Robertson JF, Aparicio S, Ellis IO, Brenton JD, Caldas C. A gene-expression signature to predict survival in breast cancer across independent data sets. Oncogene. 2007;26:1507–16. doi: 10.1038/sj.onc.1209920. [DOI] [PubMed] [Google Scholar]
- Lupu R, Menendez JA. Targeting fatty acid synthase in breast and endometrial cancer: An alternative to selective estrogen receptor modulators? Endocrinology. 2006;147:4056–4066. doi: 10.1210/en.2006-0486. [DOI] [PubMed] [Google Scholar]
- Liaw A, Wiener M. Classification and regression by randomForest. R News. 2002;2:18–22. [Google Scholar]
- Weighted Random Forests http://www.stat.berkeley.edu/~breiman/RandomForests/cc_software.htm
- Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeman JJ, Bühlmann P. Analyzing gene expression data in terms of gene sets: methodological issues. Bioinformatics. 2007;23:980–987. doi: 10.1093/bioinformatics/btm051. [DOI] [PubMed] [Google Scholar]
- Becker KG, Hosack DA, Dennis G, Jr, Lempicki RA, Bright TJ, Cheadle C, Engel J. PubMatrix: a tool for multiplex literature mining. BMC Bioinformatics. 2003;4:61. doi: 10.1186/1471-2105-4-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobl C, Boulesteix AL, Zeileis A, Hothorn T. Bias in random forest variable importance measures: illustrations, sources and a solution. BMC Bioinformatics. 2007;8:25. doi: 10.1186/1471-2105-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GeneGo MetaCore http://www.genego.com/
- Peri S, Navarro JD, Amanchy R, Kristiansen TZ, Jonnalagadda CK, Surendranath V, Niranjan V, Muthusamy B, Gandhi TK, Gronborg M, Ibarrola N, Deshpande N, Shanker K, Shivashankar HN, Rashmi BP, Ramya MA, Zhao Z, Chandrika KN, Padma N, Harsha HC, Yatish AJ, Kavitha MP, Menezes M, Choudhury DR, Suresh S, Ghosh N, Saravana R, Chandran S, Krishna S, Joy M, Anand SK, Madavan V, Joseph A, Wong GW, Schiemann WP, Constantinescu SN, Huang L, Khosravi-Far R, Steen H, Tewari M, Ghaffari S, Blobe GC, Dang CV, Garcia JG, Pevsner J, Jensen ON, Roepstorff P, Deshpande KS, Chinnaiyan AM, Hamosh A, Chakravarti A, Pandey A. Development of human protein reference database as an initial platform for approaching systems biology in humans. Genome Res. 2003;13:2363–2371. doi: 10.1101/gr.1680803. http://www.hprd.org [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Qi C, Jain S, Le Beau MM, Espinosa R, 3rd, Atkins GB, Lazar MA, Yeldandi AV, Rao MS, Reddy JK. Amplification and overexpression of peroxisome proliferator-activated receptor binding protein (PBP/PPARBP) gene in breast cancer. PNAS. 1999;96:10848–53. doi: 10.1073/pnas.96.19.10848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg R, Barnea Y, Schneebaum S, Kashtan H, Kaplan O, Skornik Y. Detection of hepatocyte growth factor/scatter factor receptor (c-Met) and MUC1 from the axillary fluid drainage in patients after breast cancer surgery. Isr Med Assoc J. 2003;5:649–52. [PubMed] [Google Scholar]
- Nacht M, Ferguson AT, Zhang W, Petroziello JM, Cook BP, Gao YH, Maguire S, Riley D, Coppola G, Landes GM, Madden SL, Sukumar S. Combining serial analysis of gene expression and array technologies to identify genes differentially expressed in breast cancer. Cancer Res. 1999;59:5464–70. [PubMed] [Google Scholar]
- Diaz LK, Wiley EL, Morrow M. Expression of epithelial mucins Muc1, Muc2, and Muc3 in ductal carcinoma in situ of the breast. Breast J. 2001;7:40–5. doi: 10.1046/j.1524-4741.2001.007001040.x. [DOI] [PubMed] [Google Scholar]
- Vgenopoulou S, Lazaris AC, Markopoulos C, Boltetsou E, Kyriakou V, Kavantzas N, Patsouris E, Davaris PS. Immunohistochemical evaluation of immune response in invasive ductal breast cancer of not-otherwise-specified type. Breast. 2003;12:172–8. doi: 10.1016/S0960-9776(03)00004-3. [DOI] [PubMed] [Google Scholar]
- Seregni E, Coli A, Mazzucca N, Italian Group RIA-IRMA Test, Italian Association of Nuclear Medicine Circulating tumour markers in breast cancer. Eur J Nucl Med Mol Imaging. 2004;31:S15–22. doi: 10.1007/s00259-004-1523-z. [DOI] [PubMed] [Google Scholar]
- Felton T, Harris GC, Pinder SE, Snead DR, Carter GI, Bell JA, Haines A, Kollias J, Robertson JF, Elston CW, Ellis IO. Identification of carcinoma cells in peripheral blood samples of patients with advanced breast carcinoma using RT-PCR amplification of CK7 and MUC1. Breast. 2004;13:35–41. doi: 10.1016/S0960-9776(03)00126-7. [DOI] [PubMed] [Google Scholar]
- Williamson EA, Wolf I, O'Kelly J, Bose S, Tanosaki S, Koeffler HP. BRCA1 and FOXA1 proteins coregulate the expression of the cell cycle-dependent kinase inhibitor p27(Kip1) Oncogene. 2006;25:1391–9. doi: 10.1038/sj.onc.1209170. [DOI] [PubMed] [Google Scholar]
- Kang HJ, Kim HJ, Kim SK, Barouki R, Cho CH, Khanna KK, Rosen EM, Bae I. BRCA1 modulates xenobiotic stress-inducible gene expression by interacting with ARNT in human breast cancer cells. J Biol Chem. 2006;281:14654–62. doi: 10.1074/jbc.M601613200. [DOI] [PubMed] [Google Scholar]
- Bos R, van der Groep P, Greijer AE, Shvarts A, Meijer S, Pinedo HM, Semenza GL, van Diest PJ, van der Wall E. Levels of hypoxia-inducible factor-1alpha independently predict prognosis in patients with lymph node negative breast carcinoma. Cancer. 2003;97:1573–81. doi: 10.1002/cncr.11246. [DOI] [PubMed] [Google Scholar]
- Gruber G, Greiner RH, Hlushchuk R, Aebersold DM, Altermatt HJ, Berclaz G, Djonov V. Hypoxia-inducible factor 1 alpha in high-risk breast cancer: an independent prognostic parameter? Breast Cancer Res. 2004;89:375–833. doi: 10.1186/bcr775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming FJ, Hill AD, McDermott EW, O'Higgins NJ, Young LS. Differential recruitment of coregulator proteins steroid receptor coactivator-1 and silencing mediator for retinoid and thyroid receptors to the estrogen receptor-estrogen response element by beta-estradiol and 4-hydroxytamoxifen in human breast cancer. J Clin Endocrinol Metab. 2004;89:375–83. doi: 10.1210/jc.2003-031048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This file contains supporting information for this paper. These include: a study of weighted random forests vs. balanced random forests, GeneGo MetaCore output and tight clusters and absolute differences results.
Tight Clustering results for 5, 10, 15 and 20 tight clusters.
Tight Clustering results for 20, 25, 30 and 35 tight clusters.