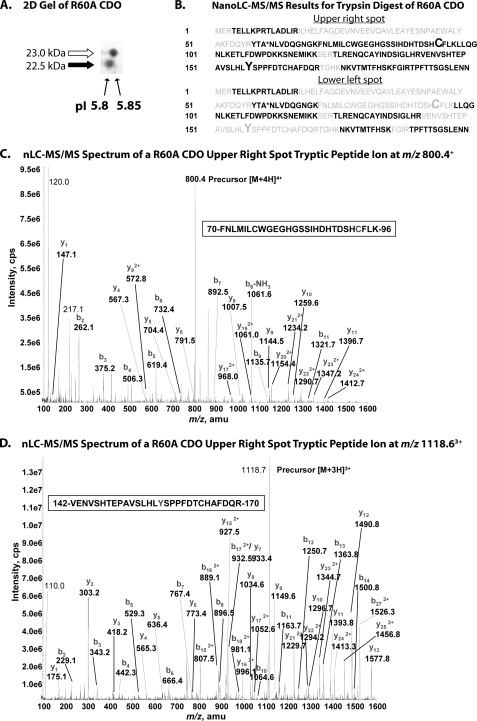
FIGURE 3.
A, two-dimensional gel (isoelectric focusing, SDS-PAGE) analysis of recombinant R60A CDO illustrating differences in pI between the upper and lower band species of CDO protein. B, composite summaries of amino acid sequences identified in peptide fragments of R60A CDO generated by trypsin digestion of the upper right and lower left spots resolved in A followed by nanoLC-MS/MS analysis. Amino acid residues positively identified in peptide fragments are indicated in black, and those that were not detected are indicated in gray. Residues Cys93 and Tyr157 are indicated in an enlarged font. The mutated residue (R60A) is indicated by an asterisk. C, MS/MS spectrum of the doubly charged peptide (FNLMILCWGEGHGSSIHDHTDSHCFLK) ion, acquired from nanoLC-MS/MS analysis of tryptic digest of R60A CDO upper band. MS/MS spectrum showed that both Cys76 and Cys93 residues are carboxyamidomethyl cysteine. D, MS/MS spectrum of the doubly charged peptide (VENVSHTEPAVSLHLYSPPFDTCHAFDQR) ion, acquired from nanoLC-MS/MS analysis of tryptic digest of R60A CDO upper band. The Cys164 residue is carboxyamidomethyl cysteine. Abbreviations used are as follows: cps, counts/s; amu, atomic mass unit.