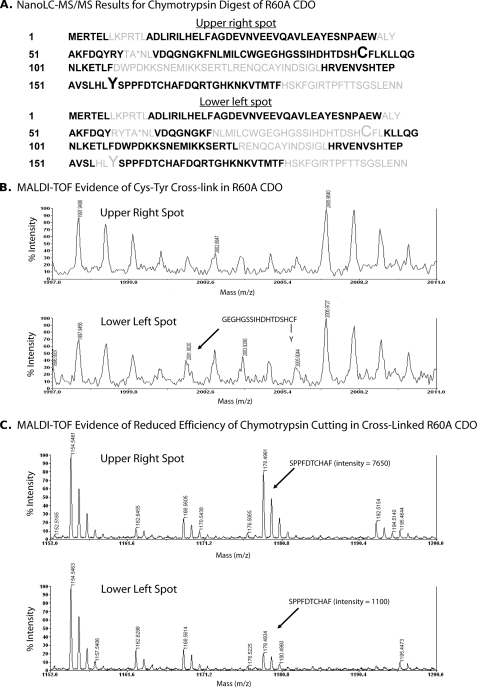
FIGURE 4.
A, composite summary of amino acid sequences identified in peptide fragments of R60A CDO generated by chymotrypsin digestion of the upper right and lower left spots from two-dimensional gel electrophoresis followed by nanoLC-MS/MS analysis. Amino acid residues positively identified in peptide fragments are indicated in black, and those that were not detected are indicated in gray. Residues Cys93 and Tyr157 are indicated in an enlarged font. The mutated residue (R60A) is indicated by an asterisk. B, MALDI evidence for the presence of a Cys93–Tyr157 cross-linked peptide in the lower left spot but not in the upper right spot of R60A CDO. C, MALDI data indicating a reduced cutting efficiency of chymotrypsin in the lower left spot of R60A CDO at the covalent bond between Tyr157 and Ser158, resulting in a poor yield of the SPPFDTCHAF peptide compared with the yield for the upper right spot of R60A CDO.