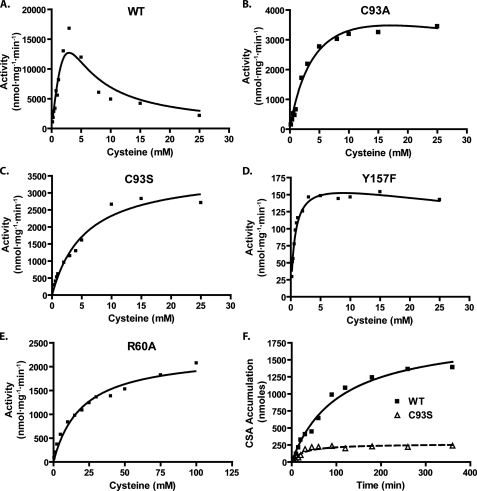
FIGURE 5.
Effect of various point mutations on CDO activity and catalytic half-life. Cysteinesulfinic acid (CSA) production was measured at various cysteine concentrations for the following purified recombinant proteins: WT (A), C93A (B), C93S (C), Y157F (D), and R60A (E). The final concentration of protein used for the activity assay experiments was as follows: WT, 1 μm; C93A/C93S, 5 μm; Y157F, 100 μm; R60A, 10 μm. Curve fitting was done using Prism4 software (GraphPad) and an integrated algorithm of the Michaelis-Menten equation for substrate inhibition. F, evaluation of the linearity of cysteinesulfinic acid production over time for the WT and C93S CDO proteins. For this last series of activity assays, cysteine was used at a final concentration of 7.5 mm, and the final concentration of CDO protein used was 1 μm WT and 4 μm C93S. The curve fit to the figure in F was done with hyperbolic regression analysis using Prism4. All assays were conducted in MES buffer, pH 6.1, with 0.3 mm ferrous iron.