Old yellow enzyme from Trypanosoma cruzi, has been crystallized using the hanging-drop vapour-diffusion method.
Keywords: old yellow enzyme, NADPH oxidoreductases
Abstract
Old yellow enzyme (OYE) is an NADPH oxidoreductase that contains a flavin mononucleotide as a prosthetic group. The OYE from Trypanosoma cruzi, which produces prostaglandin F2α, a potent mediator of various physiological and pathological processes, from prostaglandin H2. The protein was recombinantly expressed and purified from Escherichia coli and was crystallized using the hanging-drop vapour-diffusion method. The crystal belongs to the monoclinic space group P21, with unit-cell parameters a = 56.3, b = 78.8, c = 78.8 Å, β = 93.4° and two molecules per asymmetric unit. The crystals were suitable for X-ray crystallographic studies and diffracted to 1.70 Å resolution. A Patterson search method is in progress using the structure of OYE from Pseudomonas putida as a starting model.
1. Introduction
Trypanosoma cruzi is a parasitic protozoan that is transmitted to mammalian hosts by bloodsucking triatomine insects (Nakajima-Shimada et al., 1996 ▶). Infections by T. cruzi, known as Chagas disease, pose a major public health problem in the countries in Central and South America in which it is endemic (Urbina & Docampo, 2003 ▶) and result in a life-threatening, acute and/or chronic disease with severe complications. This situation is worsened by the lack of effective vaccines and the undesirable side-effects of antichagasic drugs such as nifurtimox and benznidazole, in addition to the emergence of parasite resistance to these drugs. Therefore, the development of new chemotherapeutic agents is an urgent requirement.
Chagas disease affects more than 20 million people in South America (World Health Organization, 1990 ▶). The limited success and liability of current treatments for Chagas disease has led to the search for new antitrypanosomal drugs. Naphthoquinones, such as menadione (2-methyl-1,4-naphthoquinone) and β-lapachone (3,4-dihydro-2,2-dimethyl-2H-naphtho[1,2-b]pyran-5,6-dione), and nitroheterocycles, including 4-nitroquinoline-N-oxide, nifurtimox {3-methyl-N-[(5-nitro-2-furanyl)-methylene]-4-thiomorpholinamine 1,1-dioxide} and benznidazole [2-nitro-N-(phenylmethyl)-1H-imidazole-1-acetamide], have been used for several decades as trypanocidal drugs (Aldunate & Morello, 1993 ▶; Henderson et al., 1988 ▶; Docampo & Moreno, 1986 ▶; Docampo, 1990 ▶). These compounds are thought to exert their trypanocidal action by the generation of free radicals through a redox-cycling process in which the drugs are enzymatically reduced to form drug anion radicals. Under aerobic conditions these radicals non-enzymetically reduce oxygen, leading to drug regeneration and the formation of superoxide radical anions that cause oxidative stress and cell death in susceptible parasites. Several studies have reported free-radical production upon the addition of these drugs to either intact cells or lysates of T. cruzi (Docampo & Stoppani, 1979 ▶; Boveris, Docampo et al., 1978 ▶; Boveris, Stoppani et al., 1978 ▶; Viode et al., 1999 ▶). Although naphthoquinones and nitroheterocyclic drugs have been shown to undergo the redox-cycling process within the parasite, the precise mechanism by which the drugs act and the involvement of parasite molecules in the redox-cycling process have not yet been fully elucidated.
Old yellow enzyme (OYE) was discovered in the 1930s and was first used to demonstrate the requirement of a cofactor in catalysis by enzymes (Warburg & Christian, 1933 ▶; Schopfer et al., 1991 ▶). This enzyme has since been identified in yeasts (Warburg & Christian, 1933 ▶; Matthews & Massey, 1969 ▶), plants (Schaller & Weiler, 1997 ▶) and bacteria (French et al., 1996 ▶; Blehert et al., 1999 ▶), but not in animals.
The OYE from T. cruzi (TcOYE) was identified and characterized by Kubata et al. (2002 ▶), who revealed that TcOYE catalyzes prostaglandin F2α synthesis and that its catalytic activity is affected by a variety of trypanocidal drugs such as naphthoquinone and nitroheterocyclic compounds. Furthermore, the reductase activities of epimastigotes were abolished by immunoprecipitation of the TcOYE lysates with anti-TcOYE polyclonal antibody, showing that TcOYE is a key drug-metabolizing enzyme.
In order to obtain a more detailed insight into the molecular mechanism by which the drugs act, we attempted to crystallize TcOYE. Here, we report the purification and preliminary crystallographic studies of TcOYE.
2. Materials and methods
2.1. Expression and purification of recombinant TcOYE
Recombinant TcOYE was heterologously expressed in Escherichia coli as described by Kubata et al. (2002 ▶). The PCR product encoding the TcOYE open reading frame was digested with EcoRI and XhoI restriction enzymes and then cloned into the corresponding sites of the pGEX-4T-1 expression vector (GE Healthcare). The resultant expression vector was used for transformation of E. coli BL21(DE3). Transformed cells were cultured for 8–10 h in the presence of 0.5 mM isopropyl β-d-1-thiogalactopyranoside at 303 K. The E. coli BL21(DE3) culture was harvested by centrifugation, washed with phosphate-buffered saline (PBS) containing a protease-inhibitor cocktail (Roche Diagnostics), suspended in the same buffer and disrupted by sonication (6–10 bursts of 15 s each). After removal of debris by centrifugation (3000g for 15 min), the recombinant glutathione-S-transferase-TcOYE fusion protein in the supernatant was purified by affinity chromatography on glutathione-Sepharose 4B resin (GE Healthcare) according to the manufacturer’s protocol. The fusion protein bound to the glutathione-Sepharose 4B gel was cleaved from glutathione-S-transferase using thrombin (10 units per milligram of fusion protein) and eluted with PBS. The eluted fractions containing recombinant TcOYE were pooled and concentrated and further purified by gel filtration on a Hiload 16/60 Superdex 200pg column (GE Healthcare) with the same buffer. The resulting recombinant TcOYE was then dialyzed in 20 mM sodium phosphate buffer pH 7.0 and applied onto a strong anion-exchange column (POROS HQ/M, PerSeptive Biosystems) that had been equilibrated in the same buffer. The protein was eluted with an increasing linear gradient of 0–500 mM NaCl in the same buffer. Fractions containing recombinant TcOYE were pooled, concentrated into 20 mM sodium phosphate buffer pH 7.0 and stored at 277 K. The purity of the protein was assessed by SDS–PAGE on 12%(w/v) gels according to Laemmli (1970 ▶) and the gels were stained with Coomassie Brilliant Blue (Daiichi Pure Chemicals) (Fig. 1 ▶). The protein concentration was determined using bicinchinonic acid reagent (Pierce Chemical) with BSA as a standard according to the manufacturer’s protocol.
Figure 1.
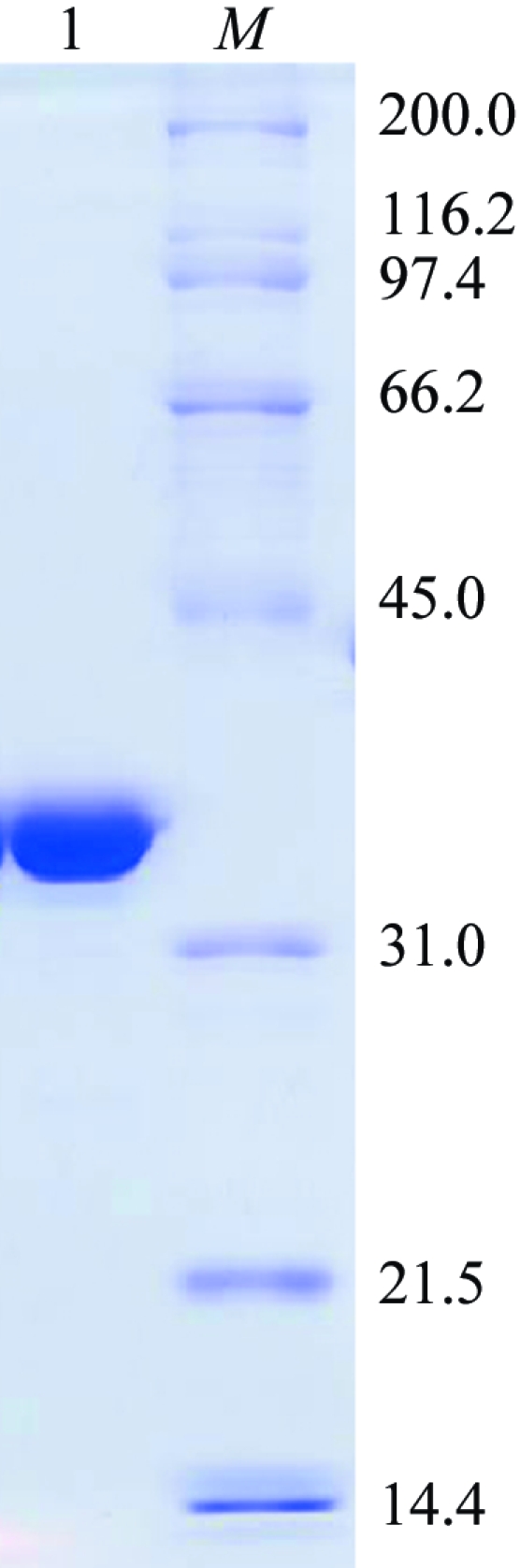
A 12% SDS–PAGE gel stained with Coomassie Brilliant Blue showing the apparent homogeneity of the purified TcOYE. Lane 1, purified TcOYE; lane M, molecular-weight markers (kDa).
2.2. Dynamic light scattering
Dynamic light-scattering studies on the recombinant TcOYE were performed using a DynamicPro-801 dynamic light-scattering instrument (Wyatt Technology Co., Inc.). Protein samples at both 10 and 15 mg ml−1 were prepared in solutions of 20 mM sodium phosphate buffer pH 7.0. Prior to the experiment, all protein samples were filtered through 0.1 µm Anotop filters (Whatman) to eliminate any aggregated particles. The reported values are averages of ten scans of 30 s each.
2.3. Crystallization
Preliminary crystallization screening conditions were constructed using the ‘Crystal T.B.’ crystallization-condition search system (Fujitsu Kyushu System Engineering Ltd, Fukuoka, Japan). The protein was concentrated to 20 mg ml−1 with a Centricon-10 concentrator. Crystals were grown by hanging-drop vapour diffusion at 293 K using equal amounts (1 µl) of protein solution and reservoir solution. Each hanging drop was placed over 0.5 ml reservoir solution.
2.4. X-ray data collection
Data collection was carried out at the SPring-8 synchrotron-radiation source (Hyogo, Japan) using an ADSC Quantum 315 detector. The crystals were soaked in reservoir solution as a cryoprotectant and then mounted in a nylon loop. The crystal was directly flash-cooled in a stream of cold nitrogen gas at 100 K. For data collection, the crystal-to-detector distance, oscillation range and exposure time were set to 180 mm, 1° and 2 s per frame, respectively. Diffraction data were processed and scaled using the HKL-2000 program package (Otwinowski & Minor, 1997 ▶).
3. Results and discussion
SDS–PAGE analysis showed a homogeneous preparation with a molecular weight of 42 kDa. Using dynamic light scattering, we found that in the presence of 0.1 M NaCl a 20 mg ml−1 solution of TcOYE is monodisperse and has an estimated molecular weight of 39 kDa, indicating the presence of monomeric TcOYE in this solution.
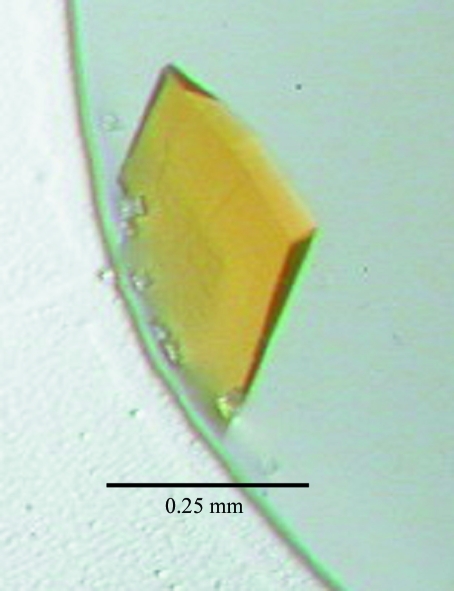
TcOYE crystals were grown by the hanging-drop method at 293 K using 24-well Cryschem plates (Hampton Research). The crystallization solutions were prepared by mixing equal volumes of protein and reservoir solutions. Preliminary crystallization trials were conducted at 293 K using the ‘Crystal T.B.’ crystallization-condition search system. Protein solution containing 20 mg ml−1 protein and 20 mM sodium phosphate buffer pH 7.0 was mixed with an equal volume of reservoir solution containing 28%(w/v) polyethylene glycol 1500 and 0.4 M ammonium fluoride. Polycrystals appeared within two weeks. After optimization of the crystallization condition using Additive Screen (Hampton Research), suitable crystals for X-ray diffraction measurement (Fig. 2 ▶) were obtained by addition of 10 mM urea to the hanging drop.
Figure 2.
Crystal of TcOYE prepared by the hanging-drop vapour-diffusion method.
Crystals of TcOYE diffracted to 1.70 Å resolution at the synchrotron-radiation source. Data-collection statistics are summarized in Table 1 ▶. The crystals belong to the monoclinic space group P21, with unit-cell parameters a = 56.3, b = 78.8, c = 78.8 Å, β = 93.4°. The packing density of the crystal, V M, is calculated to be 2.08 Å3 Da−1, which is well within the range normally found for protein crystals (Matthews, 1968 ▶). Calculation of the Matthews coefficient suggested that two monomers exist in the asymmetric unit, with a solvent content of 41%. The completeness of the intensity data was 100.0% to 1.70 Å resolution. The R merge value was 7.0% for the intensity data.
Table 1. Data-collection statistics for TcOYE.
Values in parentheses are for the highest resolution shell.
| Space group | P21 |
| Unit-cell parameters (Å, °) | a = 56.3, b = 78.8, c = 78.8, β = 93.4 |
| VM† (Å3 Da−1) | 2.08 |
| VS‡ (%) | 40.8 |
| Z | 4 |
| Resolution (Å) | 1.70 (1.76–1.70) |
| No. of observations | 424638 |
| No. of unique reflections | 75469 |
| Completeness (%) | 100.0 (100.0) |
| Rmerge§ (%) | 7.0 (27.4) |
| Redundancy | 5.6 (5.6) |
| I/σ(I) | 42.5 (5.8) |
V M = V c/ZM, where V c is the unit-cell volume and M is the molecular weight.
Solvent content V S = 1 – 1.23/V M.
R
merge = 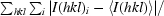
 .
.
Acknowledgments
The authors express their appreciation to Professor B. K. Kubata and Dr Z. Kabututu, Osaka Bioscience Institute, for their early support of protein expression. The authors are grateful to M. Tang, K. Miura, M. Yamashita, E. Yamashita and A. Nakagawa at SPring-8 beamlines 12B2, 40B2 and 44XU for fundamental data collection, and to M. Kawamoto, N. Shimizu and K. Hasegawa for their kind support during data collection at SPring-8 beamline 41XU. This work was supported by Grants-in-Aid for Scientific Research (Nos. 16017260 and 18350086) from the Ministry of Education, Culture, Sports, Science and Technology of Japan and the PRESTO project, Japan Science and Technology Agency, the National Project on Protein Structural and Functional Analyses, Japan (to TI) and the Center of Excellence (21COE) program ‘Creation of Integrated EcoChemistry of Osaka University’ (to KT) and was partly supported by the Applied Research Pilot Project for the Industrial Use of Space promoted by JAXA and Japan Space Utilization Promotion Center (JSUP) and a grant from Japan Foundation for Applied Enzymology and Osaka City (to YU).
References
- Aldunate, J. & Morello, A. (1993). Free Radicals in Tropical Diseases, edited by O. I. Aruoma, pp. 137–166. Chur, Switzerland: Harwood Academic Publishers.
- Blehert, D. S., Fox, B. G. & Chambliss, G. H. (1999). J. Bacteriol.181, 6254–6263. [DOI] [PMC free article] [PubMed]
- Boveris, A., Docampo, R., Turrens, J. F. & Stoppani, A. O. (1978). Biochem. J.175, 431–439. [DOI] [PMC free article] [PubMed]
- Boveris, A., Stoppani, A. O., Docampo, R. & Cruz, F. S. (1978). Comput. Biochem. Physiol. C, 61, 327–329. [DOI] [PubMed]
- Docampo, R. (1990). Chem. Biol. Interact.73, 1–27. [DOI] [PubMed]
- Docampo, R. & Moreno, S. N. (1986). Fed. Proc.45, 2471–2476. [PubMed]
- Docampo, R. & Stoppani, A. O. (1979). Arch. Biochem. Biophys.197, 317–321. [DOI] [PubMed]
- French, C. E., Nicklin, S. & Bruce, N. C. (1996). J. Bacteriol.178, 6623–6627. [DOI] [PMC free article] [PubMed]
- Henderson, G. B., Ulrich, P., Fairlamb, A. H., Rosenberg, I., Pereira, M., Sela, M. & Cerami, A. (1988). Proc. Natl Acad. Sci. USA, 85, 5374–5378. [DOI] [PMC free article] [PubMed]
- Kubata, B. K., Kabututu, Z., Nozaki, T., Munday, C. J., Fukuzumi, S., Ohkubo, K., Lazarus, M., Maruyama, T., Martin, S. K., Duszenko, M. & Urade, Y. (2002). J. Exp. Med.196, 1241–1251. [DOI] [PMC free article] [PubMed]
- Laemmli, U. K. (1970). Nature (London), 227, 409–413.
- Matthews, B. W. (1968). J. Mol. Biol.33, 491–497. [DOI] [PubMed]
- Matthews, R. G. & Massey, V. (1969). J. Biol. Chem.244, 1779–1786. [PubMed]
- Nakajima-Shimada, J., Hirota, Y. & Aoki, T. (1996). Antimicrob. Agents Chemother.40, 2455–2458. [DOI] [PMC free article] [PubMed]
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol.276, 307–326. [DOI] [PubMed]
- Schaller, F. & Weiler, E. W. (1997). J. Biol. Chem.272, 28066–28072. [DOI] [PubMed]
- Schopfer, L. M., Wessiak, A. & Massey, V. (1991). J. Biol. Chem.266, 13080–13085. [PubMed]
- Urbina, J. A. & Docampo, R. (2003). Trends Parasitol.19, 495–501. [DOI] [PubMed]
- Viode, C., Bettache, N., Cenas, N., Krauth-Siegel, R. L., Chauviere, G., Bakalara, N. & Perie, J. (1999). Biochem. Pharmacol.57, 549–557. [DOI] [PubMed]
- Warburg, O. & Christian, W. (1933). Biochem. Z.266, 377–411.
- World Health Organization (1990). Wkly Epidemiol. Rec.65, 257–261.