Table 1. Data-collection, phasing and structure-refinement statistics.
Values in parentheses are for the highest resolution shell.
| Data set | Remote | Peak |
|---|---|---|
| Wavelength (Å) | 0.992 | 1.486 |
| Space group | P6222 | P6222 |
| Unit-cell parameters (Å) | a = 180.2, c = 96.0 | a = 180.2, c = 96.0 |
| Resolution range (Å) | 48–3.0 (3.16–3.0) | 48–3.0 (3.16–3.0) |
| Unique reflections | 18818 (2606) | 18886 (2686) |
| Completeness (%) | 99.9 (99.5) | 99.9 (100.0) |
| Rsym† (%) | 9.0 (34.0) | 10.5 (58.1) |
| Multiplicity | 13.0 (13.2) | 7.3 (7.4) |
| 〈I〉/〈σ(I)〉 | 22.2 (7.8) | 13.3 (3.2) |
| Figure of merit‡ (acentric/centric) | 0.746/0.819 | |
| Phasing power (30–3.0 Å) | 0.579 | |
| No. of protein atoms | 4548 | |
| No. of Ni2+ ions | 5 | |
| No. of water molecules | 43 | |
| Rcryst§ | 22.3 | |
| Rfree§ | 26.7 | |
| R.m.s.d. bonds (Å) | 0.008 | |
| R.m.s.d. angles (°) | 1.2 | |
| R.m.s.d. B of bonded atoms (Å2) | ||
| Main chain | 0.30 | |
| Side chain | 0.81 | |
| Average B factor (Å2) | 64 | |
| Wilson B factor (Å2) | 72 | |
R
sym = 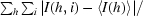
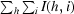 , where I(h, i) is the intensity of the ith measurement of reflection h and 〈I(h)〉 is the mean value of I(h, i) for all i measurements.
, where I(h, i) is the intensity of the ith measurement of reflection h and 〈I(h)〉 is the mean value of I(h, i) for all i measurements.
Figure of merit after phase calculation in SHARP and before solvent flattening.
R
cryst = 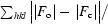
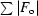 , where F
o is the observed structure-factor amplitude and F
c the calculated structure-factor amplitude. R
free is calculated based on 5% of reflections not used in refinement.
, where F
o is the observed structure-factor amplitude and F
c the calculated structure-factor amplitude. R
free is calculated based on 5% of reflections not used in refinement.
