Figure 1.
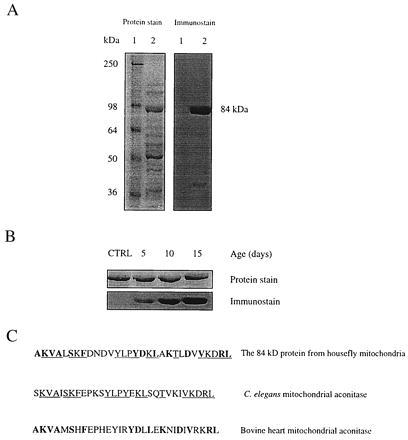
Identification of housefly mitochondrial aconitase as a target of oxidative damage. (A) Immunochemical detection of protein carbonyls in mitochondria from flight muscles of houseflies (15 days old). DNPH-treated mitochondrial matrix proteins were electrophoresed on SDS/PAGE (8.5% resolving gel) and transferred to Immobilon-P membrane. Oxidized proteins were detected immunochemically as described in the text. Lane 1 shows protein standard markers, and lane 2 contains mitochondrial matrix proteins. Protein was stained with Coomassie blue. An 84-kDa protein in the matrix exhibited a strong immunoreaction for the carbonyl groups. (B) Increase in carbonyl content of the 84-kDa protein in housefly at different ages. (Upper) The protein band stained with amido black. (Lower) The immunostain. The control (CTRL) shown is 5-day-old fly mitochondrial matrix protein without DNPH treatment. A 25-μg protein was applied onto SDS/PAGE in both A and B. (C) A computer-assisted search from protein database identified the 84-kDa protein as mitochondrial aconitase. The underlined amino acids show the N-terminal amino acid sequence homology in mitochondrial aconitase from the housefly and Caenorhabditis elegans; whereas the amino acids in bold show the homology with bovine heart mitochondrial aconitase.
