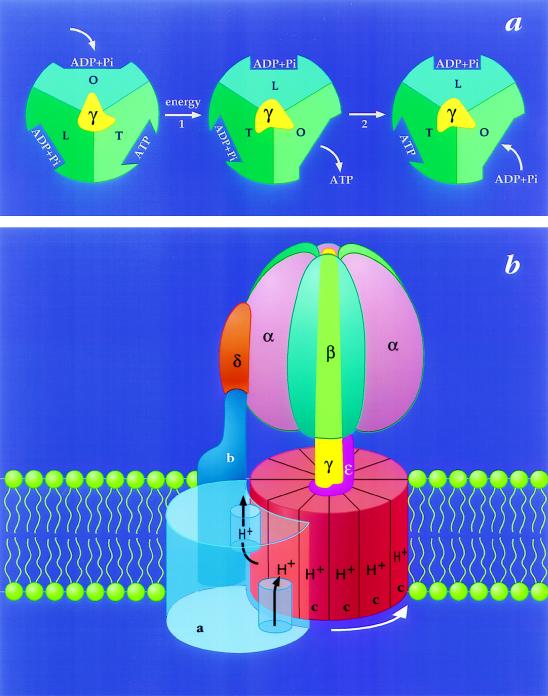
Figure 1.
The binding change mechanism for FoF1 ATP synthases. This figure was adapted from ref. 8 and modified. (a) Looking up at F1 from the membrane. In step 1, the asymmetric γ subunit rotates 120° clockwise driving conformational changes in the three catalytic sites that alter their affinities for substrates and product. In this illustration, the catalytic sites remain stationary. In step 2, ATP forms spontaneously from tightly bound ADP and Pi. For additional details and alternative views see refs. 7, 9, and 10. (b) View from the side of FoF1. The a-subunit contains two partial channels, each in contact with a different side of the membrane. In order for a H+ to traverse the membrane it moves through one channel to the center of the membrane, binds to one of the c-subunits (at Asp-61), and then is carried to the other partial channel by rotation of the c-subunit complex. The c-subunits are anchored to γ (11), whereas the a-subunit is anchored through subunits b and δ to the periphery of the α3β3 hexamer (12, 13). Hence the rotation of c-subunits relative to the a-subunit in Fo will drive the rotation of γ relative to the α3β3 hexamer in F1.