Figure 2.
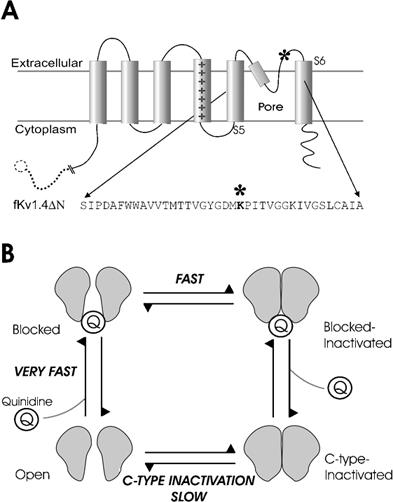
A, schematic representation of one subunit of the fKv1.4ΔN channel. The amino acid sequence of the residues in the pore-forming region and N-terminal end of S6 are shown, with an asterisk marking the approximate location of the pore-mouth lysine to tyrosine mutation at position 532. The dashed line and circle indicate the truncation of the N-terminus. B, the fundamental hypothesis of this paper is that there are two related components of quinidine block: a very fast open channel block and a slowly developing component. The slow component is a C-type inactivation that is catalysed by quinidine. This is illustrated schematically by the cartoon, which shows that the open channel usually inactivates slowly via regular C-type inactivation, but when quinidine is bound it inactivates more rapidly.
