Figure 4. Contribution of sarcolemmal transport mechanisms to decay of [Ca2+]i.
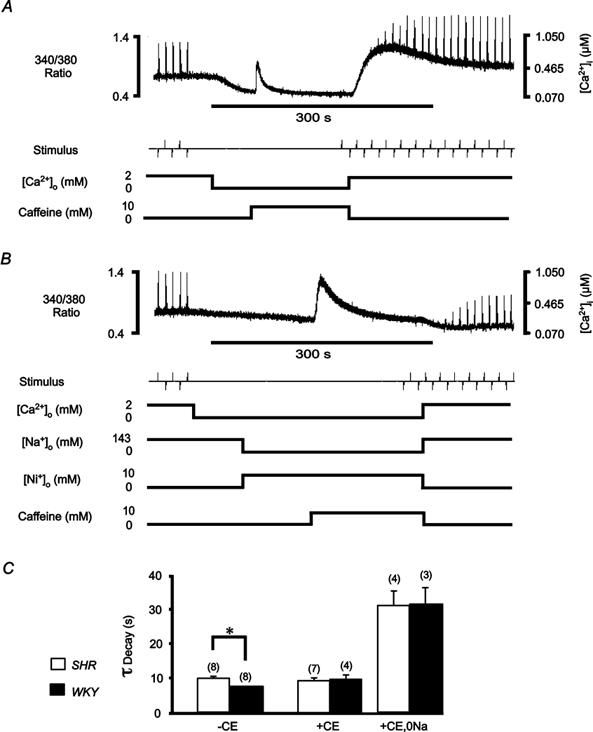
Quiescent trabeculae were exposed to 10 mm caffeine (0 mm[Ca2+]o, 22 °C) in order to functionally eliminate the SR Ca2+-ATPase. Trabeculae were stimulated at 0.1 Hz (2 mm[Ca2+]o) before and after caffeine application. An exponential function was fitted to the decay of the fluorescence ratio, from the peak of the caffeine transient, in order to determine the time constant. A, continuous recording (500 s) of the emitted 340 nm/380 nm fluorescence ratio before, during and after caffeine application in a representative LV trabecula from SHR. Following withdrawal of caffeine, the fluorescence ratio indicates a large increase in the resting [Ca2+]i that slowly decreases back to the pre-caffeine level (complete recovery not shown). B, continuous recording of the fluorescence ratio before, during and after application of caffeine in the same trabecula as in A, subsequent to loading with carboxyeosin (CE), and in the presence of 10 mm Ni+ and 0 mm Na+. Note that on withdrawal of caffeine, the fluorescence ratio shows no rapid increase in resting [Ca2+]i, suggesting that the Na+-Ca2+ exchanger was completely blocked during the caffeine application, in contrast to A. C, mean ± s.e.m. time constants (τ) of fluorescence decay during caffeine application in 0 mm Ca2+ solution (143 mm Na+) before (-CE) and after (+CE) inhibiting the SL Ca2+-ATPase and after blocking both SL transport mechanisms (+CE, 0 Na). Numbers of trabeculae as indicated in parentheses. *P < 0.05.
