Abstract
We studied somatic exocytosis of serotonin and its mediation by L-type calcium (Ca2+) channels in cultured Retzius neurones of the leech. Exocytosis was induced by trains of impulses at different frequencies or by depolarisation with 40 mm potassium (K+), and was quantified by use of the fluorescent dye FM 1–43. Stimulation increased the membrane fluorescence and produced a pattern of FM 1–43 fluorescent spots of 1.28 ± 0.01 μm in diameter, provided that Ca2+ was present in the bathing fluid. Individual spots lost their stain during depolarisation with 40 mm K+. Electron micrographs showed clusters of dense core vesicles, some of which were in contact with the cell membrane. Presynaptic structures with clear vesicles were absent from the soma. The number of fluorescent spots per soma, but not their diameter or their fluorescence intensity, depended on the frequency of stimulation. Trains at 1 Hz produced 19.5 ± 5 spots per soma, 77.9 ± 13.9 spots per soma were produced at 10 Hz and 91.5 ± 16.9 spots per soma at 20 Hz. Staining patterns were similar for neurones in culture and in situ. In the presence of the L-type Ca2+ channel blocker nimodipine (10 μm), a 20 Hz train produced only 22.9 ± 6.4 spots per soma, representing a 75 % reduction compared to control cells (P < 0.05). Subsequent incubation with 10 mm caffeine to induce Ca2+ release from intracellular stores increased the number of spots to 73.22 ± 12.5. Blockers of N-, P-, Q- or invertebrate Ca2+ channels did not affect somatic exocytosis. Our results suggest that somatic exocytosis by neurones shares common mechanisms with excitable endocrine cells.
In addition to releasing transmitter at synapses, certain neurones can secrete transmitters from the soma (Chen et al. 1995; Huang & Neher, 1996; Zaidi & Matthews, 1997, 1999; Jaffe et al. 1998). Somatic exocytosis occurs at sites where dense core vesicles fuse at extrasynaptic sites (Puopolo et al. 2001), suggesting that the mechanism of somatic secretion is different from that in synapses but similar to that in endocrine cells (reviewed by Mansvelder & Kits, 2000). Whereas synaptic secretion is typically mediated by the activation of P-, Q- and N-type Ca2+ channels (Reuter, 1996), secretion in excitable endocrine cells depends mostly on L-type Ca2+ channels (Mansvelder & Kits, 2000). In this study we analysed somatic secretion of serotonin and its mediation by L-type Ca2+ channels.
We used Retzius neurones from the CNS of the leech, which are the major serotonin producers of the animal (McAdoo & Coggeshall, 1976). Individual adult Retzius neurones can be isolated and kept in culture, where they preserve their electrical properties and continue to synthesise and release serotonin (Henderson, 1983), which is stored in clear and dense core vesicles (Kuffler et al. 1987; Bruns et al. 2000). In these cultured neurones, the events underlying synapse formation and synaptic transmitter secretion have been studied step by step (Fernández de Miguel & Drapeau, 1995). Upon electrical stimulation, serotonin is released in a quantal manner from synaptic clear and dense core vesicles (Henderson et al. 1983; Bruns & Jahn, 1995) and from somatic dense core vesicles (Bruns et al. 2000). These results suggest that somatic secretion could be functional in these neurones.
Since the somata of Retzius neurones have a low density of voltage-activated Ca2+ channels when compared with presynaptic regions (Fernández de Miguel et al. 1992; Cooper et al. 1992), the Ca2+ threshold of exocytosis may depend on the electrical activity pattern, as in excitable endocrine cells (Thomas et al. 1990; Ämmäläet al. 1993; Heinemann et al. 1993). In addition, recordings made from single Ca2+ channels (Bookman & Liu, 1990) and the analysis of Ca2+ transients (Beck et al. 2001) suggest that L-type channels are, at least in part, the pathway of somatic Ca2+ entry. Accordingly, the soma of a Retzius neurone may constitute a secretory compartment that shares common features with those observed in excitable endocrine cells.
Somatic exocytosis was analysed in single neurones by the load and release of FM 1–43 dye during stimulation (Betz et al. 1992). Secretion was induced by intracellular stimulation to produce trains of 10 action potentials at different frequencies or by increasing the external potassium (K+) concentration to 40 mm. The subcellular elements responsible for somatic exocytosis were analysed from electron micrographs; the contribution of L-type channels was studied using Ca2+ channel blockers.
METHODS
Isolation and culture of neurones
Retzius neurones were isolated from the central nervous system of adult leeches Hirudo medicinalis. The procedure has been described elsewhere (Dietzel et al. 1986). In brief, leeches were anaesthetised by immersion in 8 % ethanol. Nerve cords were dissected and ganglion capsules were opened to expose the cell somata. Ganglia were kept in Leibovitz L-15 culture medium, supplemented with 6 mg ml−1 glucose, 0.1 mg ml−1 gentamicin and 2 % heat-inactivated fetal calf serum and they were incubated for 1 h in 2 mg ml−1 collagenase/dispase solution (Boehringer-Mannheim, Mannheim, Germany). After enzyme treatment, Retzius neurones were sucked out one by one and rinsed several times in L-15 to sterilise them. Individual neurones were plated on glass culture dishes precoated with concanavalin A. Experiments were performed after 1–8 days in culture.
Stimulation of secretion
Exocytosis was analysed using the incorporation of the fluorescent dye FM 1–43 (Molecular Probes; Betz et al. 1992). FM 1–43 (2 μm) was added to the bath after neurones had been impaled and hyperpolarised to −60 mV to avoid spontaneous firing. Neurones were stimulated 3 min later. One protocol for stimulation consisted of trains of 10 action potentials produced by intracellular injection of 10 ms current pulses at 1, 10 or 20 Hz, using borosilicate microelectrodes with resistances of 18–25 MΩ when filled with 3 m KCl. Electrical recordings were acquired by an analogue-to-digital board Digidata 1200 (Axon Instruments) at a sampling frequency of 20 kHz using pCLAMP8 software (Axon Instruments) and stored in a PC. Before withdrawing the microelectrode, the dye was washed out for 2 min with physiological saline solution (mm: NaCl 120; KCl 4; CaCl2 2; Tris-maleate 10; N-methyl-d-glucamine 66) in the absence of Ca2+, which was replaced by magnesium (2 mm Mg2+) to reduce secretion (Mg2+ solution). This solution was preferred over that with an increase in the Mg2+ concentration because in raised Mg2+ solution the action potentials of Retzius neurones deteriorate (Henderson et al. 1983) and the non-specific background fluorescence increases. In Mg2+ solution, the action potentials of the neurones were reversibly prolonged but neurones remained healthy. Similarly, to test the Ca2+ dependence of secretion we used 2 mm Mg2+ instead of Ca2+ in the external solution. N-methyl-d-glucamine was added to adjust the osmolarity to 330 mosmol l−1. After the electrode was withdrawn, the cells were washed for 8 min with normal Ringer solution.
In a second protocol, exocytosis was stimulated by depolarisation with 40 mm K+. For this, FM 1–43 was added to plates containing 1 ml of normal Ringer solution and was followed by the addition of 1 ml of modified Ringer solution in which 76 mm NaCl was replaced by KCl by equimolar substitution. After 5 min, the dye was washed out as described in the previous section. Some neurones were stimulated in Mg2+ solution. Other neurones were incubated with FM 1–43 in normal (1.8 mm)-Ca2+ Ringer without stimulation or with 40 mm K+ solution in the absence of FM 1–43. The recording chamber was perfused by gravity feed; complete solution changes required 30 s.
In FM 1–43 destaining experiments, sequential images of fluorescent spots were obtained during perfusion, first with Mg2+-Ringer solution and then with another solution containing 40 mm K+ and 10 mm Ca2+ (40mm K+, 10 mm Ca2+ solution). The light intensity of individual spots was measured in all the sequential images as described below.
For Ca2+ channel blockade, nimodipine (Sigma, St Louis, MO, USA) or N-, P-, Q- and insect-type Ca2+ channel toxin blockers (Alomone Labs., Jerusalem, Israel) were added to the bath solution before addition of FM 1–43.
Analysis of exocytosis
To analyse exocytosis, individual neurones were viewed with a Nikon Eclipse TE 200 microscope through a Nikon ×100 oil immersion objective (NA 1.25). Neutral density filters reduced the illumination intensity by 90 % to reduce photobleaching of the dye and neuronal damage. Fluorescence imaging of FM 1–43-stained cells was performed with band-pass filters for excitation (peak 480 nm) and emission (peak 535 nm). Images were acquired manually, with a CCD camera (Hamamatsu Photonics, Japan) coupled to an Argus 10 integrator (Hamamatsu Photonics) programmed to integrate from 128 to 256 images each time.
Sequential images were acquired manually, approximately every 5 s and were stored digitally using Metamorph software (Universal Imaging Corp., Downingtown, PA, USA) Fluorescence was measured from manually depicted regions containing equatorial images of the cell membrane, using Metamorph software. For fluorescence measurements, the software was calibrated using the minimum and maximum fluorescence intensity regions of each cell as the 0 and 255 arbitrary 8 bit light unit (u) values, respectively. Fluorescence was measured by linear interpolation. For background subtraction, fluorescence was measured from a region containing no cell. The light intensity of this region in each sequential image was subtracted from the intensity of the membrane in the same image. Fluorescence values were normalised to the initial value of each cell for comparisons. Because of the focal distance, we expect that the total number of fluorescent spots was underestimated in focal planes distant from the objective.
After washout of FM 1–43 from the external medium, neurones displayed a characteristic staining pattern of fluorescent spots. To analyse this pattern, whole neurones were imaged in z series under calibrated conditions and the number of fluorescent spots per soma was manually quantified from the sequential focal planes using Metamorph software and applying stereological criteria (Coggeshall & Lekan, 1996). The quantitative analyses of the light intensity, and the spot diameters were made from confocal (Bio-Rad) serial z images taken at 1.0 μm intervals using the fluorescein filters already described. The major diameter of the spots was measured manually, tracing a straight line across it. For a more precise calibration of measurements in the confocal images, we used yellow- green- or red-fluorescent carboxylate-modified microspheres of 2.0 and 0.5 μm diameter (FluoSpheres, Molecular Probes).
Electron microscopy
To analyse the subcellular elements participating in secretion, non-stimulated cultured neurones were washed with 0.08 m cacodylate buffer (Sigma) and fixed for 10 min with 0.6 % glutaraldehyde and 0.4 % paraformaldehyde in 0.08 m cacodylate buffer, pH 7.4 (Kuffler et al. 1987). Postfixation was performed in 1 % osmium tetroxide (Fluka, St Louis, MO, USA) in cacodylate buffer. Cells were serially dehydrated and infiltrated in Epon (Electron Microscopy Science, Fort Washington, PA, USA):ethanol (1:1) overnight. After several substitutions of Epon, the blocks were polymerised at 60 °C for 24 h. Ultra thin sections were counterstained with uranyl acetate for 10 min followed by lead citrate for 2.5 min. Thin sections were observed in a Jeol 1010 electron microscope (Jeol USA Inc., Peabody, MA, USA). In some cases, to compare the morphology of somatic and synaptic release sites, Retzius neurones were plated in close apposition to pressure sensory neurones upon which they form chemical synapses (Fuchs et al. 1982).
Clusters of vesicles were measured manually, tracing a straight line across their largest diameter. Clusters in which vesicles were closer than 150 nm to the plasma membrane were considered superficial. Clusters with vesicles located more than 150 nm away from the membrane were classified as internal clusters.
Statistical analysis
Data are expressed as mean values ± the standard error of the mean (s.e.m.). Mean values of the diameters of the fluorescence spots and vesicle clusters were calculated from Gaussian fits to the data. For statistical analysis, one-way analysis of variance was used to compare the means of more than two groups. If a significant difference was found, a group-by-group comparison was performed using Student's unpaired t test. Two groups were compared using Student's unpaired t test. P < 0.05 was considered significant.
RESULTS
Somatic secretion in retzius neurones
Stimulation of Retzius neurones with microelectrodes in the presence of FM 1–43 produced a gradual fluorescence increase of the plasma membrane area. Figure 1 shows a phase contrast image of a neurone (Fig. 1A), the train of action potentials in response to intracellular stimulation (Fig. 1B) and subsequent fluorescent images of a soma area of the same neurone before and after a 20 Hz train of impulses (Fig. 1C). The rim of membrane seen in Fig. 1C shows occasional extracellular patches of fluorescent debris stained with FM 1–43 (asterisk). This non-specific staining was excluded from our analysis (Smith & Betz, 1996), since it disappeared when neurones were washed with Mg2+ solution (see Methods). Fluorescence levels were measured only from clean membrane areas (Fig. 1C, arrowhead). Whilst before stimulation the membrane fluorescence levels were constant (Fig. 1D), stimulation produced a gradual increase in the membrane fluorescence, which after 2 min was 96 ± 20 % above the basal levels (range from 50–180 % n= 7 cells; Fig. 1D), suggesting vesicle fusion and endocytosis in the soma membrane (Smith & Betz, 1996).
Figure 1. Electrical stimulation increases somatic FM 1–43 fluorescence in Retzius neurones.
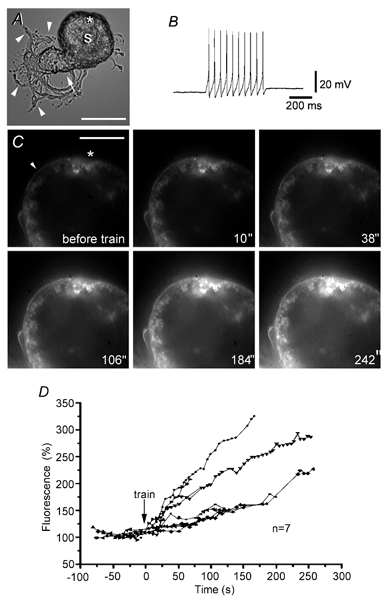
A, phase contrast micrograph of a Retzius neurone in culture showing the soma (S) attached to the stump (arrow). Neurites and growth cones are marked with arrowheads. Scale bar represents 60 μm. B, intracellular recording of the 20 Hz train produced by current injection.C, fluorescence images of a selected somatic region of the same Retzius neurone before and after the 20 Hz train in the presence of 2 μm FM 1–43. The time after stimulation is shown in each image (′′ indicates seconds). Note the gradual increase in fluorescence. The arrowhead points to a membrane area used to measure fluorescence over time. The asterisk marks a region with debris which was excluded from the analysis. Scale bar represents 30 μm. D, fluorescence increase of a selected membrane area of 7 neurones before and after stimulation. Continuous lines link symbols representing data from individual neurones.
Much of the fluorescence was reduced and disappeared during superfusion with Mg2+ solution (Fig. 2A and B). The decay of this non-specific fluorescence (Cochilla et al. 1999) unmasked an FM 1–43 staining pattern consisting of fluorescent spots distributed at the cell surface (Fig. 2A). To demonstrate that fluorescent spots could be sites for exocytosis, stained neurones were superfused with 40 mm K+, 10 mm Ca2+ solution and the light intensity of the fluorescent spots was measured over time (Fig. 2C and D). In the presence of the washing Mg2+ solution and before the depolarisation, the fluorescence intensity of the spots decreased gradually, possibly because of dye bleaching during continuous illumination. The increase of external K+ decreased fluorescence to its basal levels (n= 12 spots from three neurones; Fig. 2C and D) within the next minute. As shown in Fig. 2C and D, the fluorescence decay was gradual instead of stepwise, suggesting that each spot contained several stained vesicles.
Figure 2. Fluorescent FM 1–43 spotted pattern of Retzius neurones.
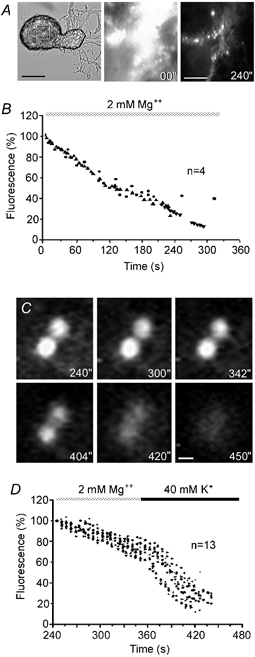
A, phase contrast of a Retzius neurone and fluorescence images of the selected area of the soma membrane at different times after electrical stimulation with a 20 Hz train in the presence of FM 1–43. Fluorescence images were taken at the beginning (00′′) and after 240 s of perfusion with Mg2+ solution. Washing the non-specific FM 1–43 staining off the membrane revealed fluorescent spots. Scale bars represent 60 μm (phase contrast) and 10 μm (fluorescence), respectively. B, decay of fluorescence in selected somatic membrane areas of 4 neurones during perfusion with Mg2+ solution to wash off non-specific FM 1–43 staining. Series of data from 4 neurones are superimposed. C, fluorescent FM 1–43 spots imaged at different times during perfusion with Mg2+ solution (top three images) and during depolarisation with 40 mm K+, 10 mm Ca2+ solution (bottom three images). Depolarisation in the presence of Ca2+ destained the fluorescent spots rapidly. The times shown are contiguous with those in A and B. Scale bar represents 1 μm. D, decay of FM 1–43 fluorescence intensity during superfusion with Mg2+ solution followed by depolarisation with 40 mm K+, 10 mm Ca2+ solution. The perfusion protocol is above. Series of data from 12 spots in 3 neurones are superimposed.
Ultrastructural analysis of possible secretion sites
A search was made in electron micrographs for the subcellular elements that produced fluorescent spots. A clear distinction could be made between the classes of vesicles found in the soma and those found in the stump of a neurone (Fig. 3). As in previous observations, the neuronal soma contained clusters of 100 nm dense core vesicles (Bruns et al. 2000; V. Hernandez, M. Morales & F. F. De-Miguel, unpublished observations), some of which were in close apposition to the plasma membrane (Fig. 3A and B). These vesicles were similar to those seen in Retzius cells in situ (Coggeshall, 1972; Yaksta-Sauerland & Coggeshall, 1973). In all six neurones studied, small (40 nm) clear vesicles were not detected in the somata, although they were common in the neuronal stump. The clusters of dense core vesicles contained a mixture of fully dense vesicles, vesicles with concentric cores and, occasionally, large (100 nm) clear vesicles.
Figure 3. Subcellular distribution of structures possibly representing secretory organelles.
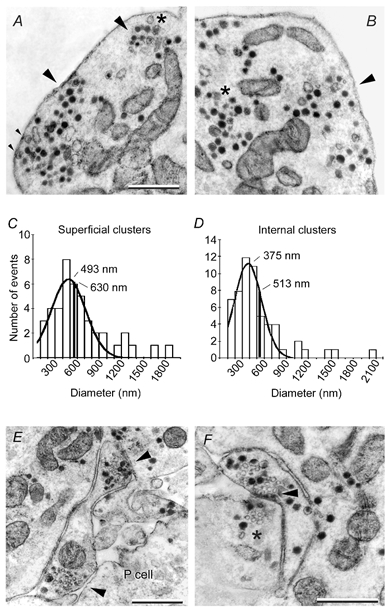
A, electron micrograph showing subcellular somatic clusters of dense core vesicles (arrowheads) and mitochondria. Clusters near the cell membrane with vesicles making contact with it are marked with small arrowheads. Presynaptic densities were absent. A large clear vesicle is marked with an asterisk. B, in addition to the vesicle clusters near the cell membrane (arrowhead) other clusters were at a distance from it (asterisk). Scale bar in A also applies to B. C, diameter distribution of superficial clusters. The continuous line is a Gaussian fit to the data with a mean value of 493 ± 35 nm. The black vertical line is the average diameter of the whole population. D, similar analysis for clusters at distances larger than 150 nm from the cell membrane. E, micrograph of the contact region with a pressure sensory (P cell) neurone showing a process of the Retzius neurone with two groups of clear and dense core vesicles near the cell membrane (arrowheads). F, autapse formed by a process and the stump of a Retzius neurone. A dense presynaptic zone with clear and dense core vesicles (arrowhead) is in close apposition to the cell membrane. A cluster of vesicles in the stump is marked with an asterisk. Scale bars represent 1 μm in all cases.
The diameter of the superficial vesicle clusters, estimated from a Gaussian fit to the diameter distribution histogram (Fig. 3C), was 493 ± 35 nm (n= 43). Since this estimate excluded the largest clusters measured, we estimated the average value of the whole population, which was 630 ± 58 nm. There were also clusters of large dense core vesicles that did not make contact with the cell membrane even in several serial sections (Fig. 3B, asterisk). The mean diameter of these clusters was estimated at 375 ± 24 nm from a Gaussian fit to the diameter distribution histogram and at 513 ± 48 nm considering the whole population (Fig. 3D). In addition to the clusters, there were scattered dense core vesicles near the membrane and within the cytoplasm.
In serial sections obtained from six neurones we did not find somatic autapses or presynaptic endings with small (30–50 nm) clear vesicles (Henderson et al. 1983; Kuffler et al. 1987) that could contribute to the punctate FM 1–43 pattern described here. Such presynaptic endings with small clear and large dense core vesicles were restricted to the neuronal stump. They were found in sites of contact with pressure sensory neurones (P cell), on which they form chemical synapses (Fig. 3E; and Henderson et al. 1983), or on the stump itself as autapses (Fig. 3F). The subcellular structure of these two types of presynaptic terminals was similar and consisted of clusters of small clear vesicles closely apposed to the plasma membrane and capped by dense core vesicles (Fig. 3E and F; and Kuffler et al. 1987). These results supported that somatic secretion occurred from dense core vesicles and raised the possibility that the fluorescent FM 1–43 spots were produced by staining of vesicle clusters.
Characteristics of the FM 1–43 staining pattern
To explore the possibility that fluorescent spots were produced by clusters of FM 1–43-loaded vesicles, we analysed the characteristics of the spots under confocal calibrated conditions. Neurones were stimulated with trains of 10 action potentials at 1 or 10 Hz, or they were depolarised for 5 min with 40 mm K+. As can be seen in the confocal three-dimensional reconstructions in Fig. 4A, the number of fluorescent spots varied according to the stimulation protocol. While a 1 Hz train produced occasional fluorescent spots, a 10 Hz train or 40 mm K+ depolarisation produced profuse spotted staining. Figure 4A also shows amplified fluorescent spots produced by each stimulation protocol and images of 0.5 and 2.0 μm beads for calibration. It is interesting to note that the fluorescent spots were asymmetric and that they had uneven light intensities, suggesting that each of them contained several vesicles. The light intensity profiles of fluorescent spots in the same neurone or in neurones stimulated with the different protocols had similar maximum peaks (Fig. 4B).
Figure 4. FM 1–43 staining patterns of Retzius neurones.
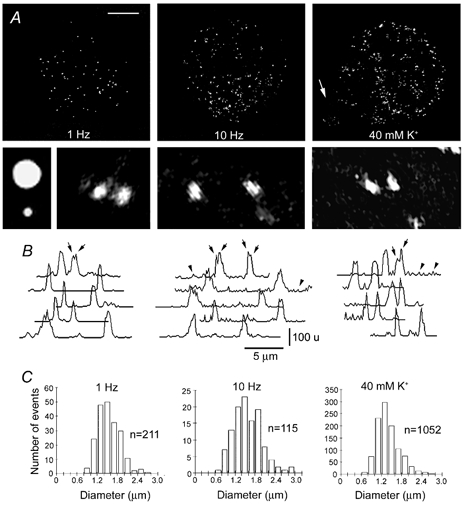
A, confocal three-dimensional (3-D) reconstructions of Retzius neurones stimulated in the presence of FM 1–43. The stimulation protocol is stated in each image. The arrow points to the stump. Scale bar represents 30 μm. Below are shown (left to right) amplified fluorescence images of fluorescent beads with 2.0 and 0.5 μm diameters and FM 1–43 spots of the neurones stimulated with each protocol. Note the asymmetries of the spots. B, light intensity profiles of several spots in each of the neurones above. The top traces correspond to the spots shown in the images. Different light intensity peaks in each spot were common (arrows). Intensities are in an arbitrary 256 unit (u) grey scale. Note the increase of the baseline noise in the profiles of neurones stimulated with 10 Hz and with high K+ (arrowheads). C, histograms of the diameter distribution of FM 1–43 spots obtained from confocal images of different cells stimulated with each protocol.
It was common that in every stimulation condition, the light intensity profiles had more than one peak (arrows in Fig. 4B), again pointing to multivesicular contents. In addition, after high frequency trains or high K+ depolarisation, the cell membrane around the spots had small fluorescence peaks that increased the baseline noise of the light intensity profiles (Fig. 4B, arrowheads), suggesting that single scattered vesicles had been stained by FM 1–43. Nevertheless, the resolution of our system limited the interpretation of this staining component and our subsequent analysis is restricted to the fluorescent spots.
In addition to the similarity of the light intensity profiles of the FM 1–43 spots, their largest diameters were similar under the different stimulation protocols (Fig. 4C). Gaussian distributions of spot diameters from neurones stimulated with trains at 1 Hz (n= 211, from two cells) and 10 Hz (n= 115, from two cells) or with 40 mm K+ (n= 1052, from seven cells) had statistically similar mean values of 1.34 ± 0.58, 1.36 ± 0.45 and 1.16 ± 0.35 μm, respectively. Since the diameter of somatic dense core vesicles is 100 nm (Bruns et al. 2000), the fluorescent spots could be produced by clusters of vesicles instead of single vesicles (Angleson et al. 1999).
Frequency and calcium dependence of FM 1–43 staining
The dependence of the number of spots on the stimulation protocol raised the possibility that the number of spots was a measure of the extent of the previous exocytosis. To test this, we counted the fluorescent spots in serial z planes of neurones stimulated with trains at 1, 10 and 20 Hz or with high K+ depolarisation. Figure 5A shows intracellular recordings of 1 and 10 Hz trains of impulses and Fig. 5B shows fluorescence non-confocal images of neurones stimulated by each different protocol. These images were taken 10 min after stimulation over the contact area of the neurones with the glass bottom of the plate. Whilst ten stimuli at 1 Hz produced 19.5 ± 5 spots per soma (n= 6), the same number of impulses at 10 Hz produced a significantly larger number of spots (77.9 ± 13.9; n= 8). Increasing the stimulus frequency to 20 Hz produced 91.5 ± 16.9 spots per soma (n= 10), statistically similar to that with 10 Hz (Fig. 5C). Neurones depolarised for 5 min with 40 mm K+ produced a significantly larger number of spots per soma (166.8 ± 16.8; n= 8) than those stimulated electrically.
Figure 5. Frequency and Ca2+ dependence of FM 1–43 staining.
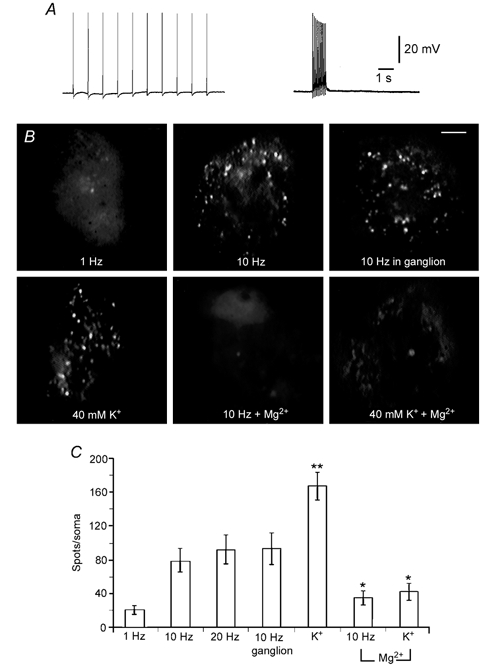
A, intracellular recordings of trains of action potentials at 1 and 10 Hz, respectively, produced by intracellular current injection. B, staining pattern of neurones stimulated with the different protocols indicated in each image. The staining patterns of neurones stimulated in culture or in the ganglion were similar. Substituting Mg2+ for Ca2+ prevented FM 1–43 staining in neurones stimulated with a 10 Hz train or with 40 mm K+. Fluorescence images are non-confocal and were taken at comparable focal planes at the site of contact with the plate. Scale bar represents 10 μm. C, quantification of the total number of spots per soma under the different stimulation conditions shown in B. * Significant (P < 0.05) differences with respect to neurones stimulated in the presence of Ca2+. The number of spots per soma in neurones depolarised with 40 mm K+ was significantly (P < 0.05) larger than in neurones stimulated with microelectrodes (**).
To test if the same FM 1–43 staining patterns occur in situ, neurones were stimulated in the ganglion with 10 Hz trains in the presence of FM 1–43. In this experiment it was essential to improve the signal to noise ratio of the images, affected by the non-specific binding of the dye in the ganglion, and also to obtain imaging conditions comparable to those in culture. For this purpose, stimulated neurones were isolated from the ganglion in Mg2+ solution to avoid secretion-induced destaining and were then plated in glass-bottom dishes. As can be seen in Fig. 5B, the FM 1–43 staining persisted throughout the isolation procedure and the fluorescent spotted pattern was similar to that produced in culture. In particular, the number of spots per soma of these neurones (92.2 ± 18.4, n= 7) was statistically similar to that of neurones stimulated with 10 Hz in culture.
When external Ca2+ was replaced by Mg2+, the number of spots per soma produced by 10 Hz trains or high K+ depolarisation in cultured neurones was significantly reduced to 34 ± 8 (n= 7) and 41.6 ± 10 (n= 5), respectively. The characteristics and number of spots obtained in these conditions were similar to those of neurones stimulated at 1 Hz. Figure 5C compares the number of spots of neurones stimulated in each different condition described in this section. Fluorescent spots were also absent from neurones incubated with FM 1–43 in the presence of extracellular Ca2+ but without depolarisation, or from neurones depolarised with 40 mm K+, 10 mm Ca2+ in the absence of FM 1–43 (not shown). Hence, the number of somatic FM 1–43 spots varied in a manner similar to that expected for Ca2+- and activity-dependent exocytosis.
L-type Ca2+ channels and somatic secretion
The frequency dependence of somatic secretion and the low density of Ca2+ channels in the somata (Fernández de Miguel et al. 1992) suggested that the mechanism of exocytosis may be similar to that of excitable endocrine cells. In such cells Ca2+ entry through L-type Ca2+ channels during repetitive firing reaches the threshold concentration for secretion (reviewed by Mansvelder & Kits, 2000). As shown in Fig. 6, a train of 10 impulses at 20 Hz in the presence of 10 μm nimodipine to block L-type Ca2+ channels (Triggle & Janis, 1987; Regan et al. 1991), produced 22.9 ± 6.4 spots per soma (n= 12), only 25 % of the number of spots in control neurones stimulated without nimodipine (91.5 ± 16.9; n= 10; Fig. 6). The number of spots in the presence of nimodipine was similar to that produced by a 1 Hz train or by a 10 Hz train in the absence of external Ca2+ (compare Fig. 5C and Fig. 6C). However, when nine of the neurones incubated with nimodipine were subsequently incubated with 10 mm caffeine to stimulate Ca2+ release from intracellular stores (Sitsapesan & Williams, 1990; Usachev et al. 1993) in the presence of FM 1–43, the number of spots increased significantly to 73.2 ± 12.5 spots per soma (Fig. 6B), statistically similar to the value in control neurones (Fig. 6C).
Figure 6. Blockade of somatic secretion by nimodipine.
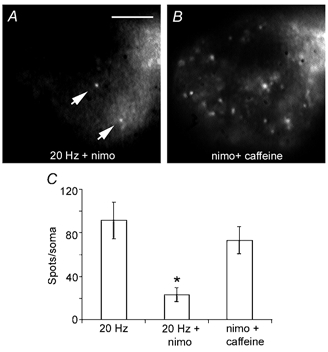
A, fluorescence image of a Retzius neurone stimulated with a 20 Hz train in the presence of 10 μm nimodipine (nimo) and FM 1–43. Only a few spots can be seen (arrows). Scale bar represents 20 μm. B, subsequent incubation with 10 mm caffeine in the presence of FM 1–43 increased the number of fluorescent spots. The focal plane of the neurone is the same as in A. Note that the spots in A are also present in B. C, number of spots produced by the stimulation conditions in A and B, compared with control neurones stimulated with a 20 Hz train.
The contributions of other Ca2+ channel types to somatic secretion were measured by depolarising neurones with 40 mm K+ in the presence of toxins that block different types of Ca2+ channels. Previous studies (Dierkes et al. 1997; Beck et al. 2001) in Retzius neurones showed that somatic Ca2+ entry remains unaffected in the presence of ω-conotoxin GVIA, or ω-agatoxin IVA and ω-agatoxin TK which block N and P/Q channels, respectively. This lack of effect could be attributed to the insensitivity to these (and most) toxins of neurones of this leech species (Johansen & Kleinhaus, 1986). We extended this analysis to another group of toxins which included: ω-conotoxin MVIIA (2 μm; n= 6), which blocks N-type channels; FTX 3.3 (1 μm; n= 8), which blocks P/Q-type Ca2+ channels; ω-conotoxin MVIIC (1 μm; n= 6), which blocks Q-type channels; and PLTX-II toxin (50 nm; n= 8), which blocks an insect-type of Ca2+ channel. Only nimodipine (20 μm; n= 7) significantly reduced (47 %) the number of fluorescent spots when compared with control neurones (Table 1).
Table 1.
Effects of different calcium channel blockers on somatic secretion
| Channel type | Blocker | Concentration | Spots per soma | n |
|---|---|---|---|---|
| L | Nimodipine | 20 μM | 88.42 ± 07.86* | 7 |
| P/Q | FTX 3.3 | 1 μM | 176.50 ± 11.83 | 8 |
| Q | ω-Conotoxin MVIIC | 1 μM | 175.00 ± 22.94 | 6 |
| N | ω-Conotoxin MVIIA | 2 μM | 183.33 ± 07.74 | 6 |
| Insect | PLTX | 50 nM | 179.62 ± 12.09 | 8 |
| Control | — | — | 166.87 ± 16.80 | 8 |
Secretion was stimulated by 40 mM K+ depolarisation. The number of spots per soma is expressed as the mean ±s.e.m.
Significant difference with respect to control.
DISCUSSION
We have shown that Retzius neurones display frequency-dependent somatic exocytosis and endocytosis. Stimulation in the presence of FM 1–43 increased the somatic membrane fluorescence, and produced a depolarisation- and Ca2+-dependent FM 1–43 spotted pattern that destained upon further depolarisation. The number of fluorescent spots was frequency dependent, with a dynamic range between 1 and 10 Hz, although the diameter and light intensity of individual spots were similar in all the stimulation conditions. Somatic secretion was dependent on L-type Ca2+ channels, since nimodipine reduced the number of fluorescent spots. Our evidence suggests that the fluorescent spots consisted of clusters of dense core vesicles.
A train of impulses in Retzius neurones evoked somatic vesicle fusion and endocytosis, as indicated by the increase in the FM 1–43 fluorescence (Smith & Betz, 1996; Kilic et al. 2001). The time course of the fluorescence increase suggests that brief stimulation may trigger long-lasting secretory and endocytic activity. A similar response has been reported in chromaffin cells, in which exocytosis and endocytosis continue for several minutes after stimulation, even when the intracellular Ca2+ concentration has returned to its basal levels (Penner & Neher, 1988; Heinemann et al. 1993).
At first glance, the well-defined fluorescent spotted pattern of Retzius neurones was similar to the FM 1–43 fluorescent pattern of lactotrophs (Angleson et al. 1999) and pancreatic acinar cells (Giovannucci et al. 1998). However, while individual spots in lactotrophs are produced by single large granules (Angleson et al. 1999), the asymmetry of individual fluorescent spots in Retzius neurones, their diameters and their light intensity profiles suggest that each spot is formed by several vesicles. The use of fluorescent beads made it clear that the size of the FM 1–43 spots was within the linear range of resolution and, therefore, could not be explained by the light emission from single 100 nm vesicles. For these reasons, each fluorescent spot seems to reflect an active zone for multiple secretory and endocytic events. This multivesicular hypothesis is also supported by the gradual destaining of the spots during depolarisation and by ultrastructural evidence of dense core vesicle clusters, also seen by Bruns et al. (2000). An open question is how the number of fluorescent spots increases with the firing frequency of the cell. Such dynamics suggest the recruitment of cytoplasmic vesicle clusters, and may be produced by the activity-dependent migration of vesicle clusters, similar to that in chromaffin cells (Steyer & Almers, 1999).
Calcium dependence of exocytosis
The frequency dependence of somatic secretion in Retzius neurones is similar to that in excitable endocrine cells (Thomas et al. 1990; Zorec et al. 1991; Ämmäläet al. 1993), in which low activity levels hardly trigger secretion because of the distance between Ca2+ channels and vesicles (Augustine & Neher, 1992). However, with high frequency trains, intracellular Ca2+ accumulates and diffuses, reaching the vesicle fusion threshold (Heinemann et al. 1993). The soma of Retzius neurones has a low density of voltage-activated Ca2+ channels when compared with the stump or with presynaptic regions (Fernández de Miguel et al. 1992). This may explain the small increases in the somatic intracellular Ca2+ concentration reported by Beck et al. (2001) and the high frequency requirements of somatic secretion.
L-type Ca2+ channels make a major contribution to somatic exocytosis in Retzius neurones, dopaminergic neurones of the substantia nigra (Puopolo et al. 2001) and excitable endocrine cells (Davalli et al. 1996; Villalobos et al. 1997). By contrast, synaptic secretion in most neurones is mediated by N- and P/Q-type channels (see for example, Reuter, 1996). However, since in Retzius neurones nimodipine blocks somatic secretion only partially during long K+-induced depolarisations, other channel types may also participate, as in excitable endocrine cells (Artalejo et al. 1994: Davalli et al. 1996; Lukyanetz & Neher, 1999). The lack of sensitivity of Retzius neurones to many channel blocker toxins (Johansen & Kleinhaus, 1986) does not allow us to exclude the participation of N-, P- and Q- channels in somatic secretion based on our negative results. In addition, the effect of caffeine to induce somatic secretion during L-type Ca2+ channel blockade suggests that Ca2+-induced Ca2+ release could be taking part in somatic secretion, as in excitable endocrine cells (Lemmens et al. 2001).
Possible functional significance of somatic secretion
The similar FM 1–43 staining patterns of Retzius neurones in culture and in the ganglion suggest that somatic secretion is physiological. The soma of Retzius neurones seems to be well suited for this function, since its large surface area allows massive serotonin secretion from large numbers of secretory vesicles in short periods. Retzius neurones display a bimodal behaviour, releasing transmitter from presynaptic terminals upon low activity levels (Henderson et al. 1983; Dietzel et al. 1986; Stewart et al. 1989; Bruns & Jahn, 1995), and secreting large amounts of transmitter from somatic dense core vesicles at high firing frequencies. Through this regulated mechanism of serotonin secretion, a single neurone type may modulate the activity of single synaptic targets (Mar & Drapeau, 1996) or whole neuronal circuits (Willard, 1981; Kristan & Nusbaum, 1982; Lent & Dickinson, 1984; Lockery & Kristan, 1990; Wilson et al. 1996).
Summarising the information available in different neurone types, the mechanism of somatic secretion is different from that in synapses but similar to the mechanism used by excitable endocrine cells. Neuronal somatic exocytosis occurs from dense core vesicles which release biogenic amines or peptides (Chen et al. 1995; Huang & Neher, 1996; Zaidi & Matthews, 1997, 1999; Jaffe et al. 1998; Puopolo et al. 2000) and it may be part of a more general mechanism for the neuromodulation of neuronal populations.
Acknowledgments
We wish to thank Dr Damien Kuffler for his advice concerning electron microscopy and Victor Hugo Hernandez, Jorge Sepúlveda and Rodolfo Paredes for processing the samples for electron microscopy. C.T. was supported by CONACYT and DGEP fellowships. Human Frontiers Science Program (RG-162/98), CONACYT (1285-N9204) and PAPIIT (IN-213196) grants to F.F.M. have supported this project.
REFERENCES
- Ämmälä C, Eliasson L, Bokvist K, Larsson O, Ashcroft FM, Rorsman P. Exocytosis elicited by action potentials and voltage-clamp calcium currents in individual mouse pancreatic B-cells. J Physiol. 1993;472:665–688. doi: 10.1113/jphysiol.1993.sp019966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angleson JK, Cochilla AJ, Kilic G, Nussinovitch I, Betz WJ. Regulation of dense core release from neuroendocrine cells revealed by imaging single exocytic events. Nat Neurosci. 1999;2:440–446. doi: 10.1038/8107. [DOI] [PubMed] [Google Scholar]
- Artalejo CR, Adams ME, Fox AP. Three types of Ca2+ channel trigger secretion with different efficacies in chromaffin cells. Nature. 1994;367:72–76. doi: 10.1038/367072a0. [DOI] [PubMed] [Google Scholar]
- Augustine GJ, Neher E. Calcium requirements for secretion in bovine chromaffin cells. J Physiol. 1992;450:247–271. doi: 10.1113/jphysiol.1992.sp019126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A, Lohr C, Deitmer JW. Calcium transients in subcompartments of the leech Retzius neuron as induced by single action potentials. J Neurobiol. 2001;48:1–18. doi: 10.1002/neu.1039. [DOI] [PubMed] [Google Scholar]
- Betz WJ, Bewick GS, Ridge RM. Intracellular movements of fluorescently labeled synaptic vesicles in frog motor nerve terminals during nerve stimulation. Neuron. 1992;9:805–813. doi: 10.1016/0896-6273(92)90235-6. [DOI] [PubMed] [Google Scholar]
- Bookman RJ, Liu Y. Analysis of Ca2+ channel properties in cultured leech Retzius cells by internal perfusion voltage-clamp and single-channel recording. J Exp Biol. 1990;149:223–237. doi: 10.1242/jeb.149.1.223. [DOI] [PubMed] [Google Scholar]
- Bruns D, Jahn R. Real-time measurement of transmitter release from single synaptic vesicles. Nature. 1995;377:62–65. doi: 10.1038/377062a0. [DOI] [PubMed] [Google Scholar]
- Bruns D, Riedel D, Klingauf J, Jahn R. Quantal release of serotonin. Neuron. 2000;28:205–220. doi: 10.1016/s0896-6273(00)00097-0. [DOI] [PubMed] [Google Scholar]
- Chen G, Gavin PF, Luo G, Ewing AG. Observation and quantitation of exocytosis from the cell body of a fully developed neuron in Planorbis corneus. J Neurosci. 1995;15:7747–7755. doi: 10.1523/JNEUROSCI.15-11-07747.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochilla AJ, Angleson JK, Betz WJ. Monitoring secretory membrane with FM 1–43 fluorescence. Ann Rev Neurosci. 1999;22:1–10. doi: 10.1146/annurev.neuro.22.1.1. [DOI] [PubMed] [Google Scholar]
- Coggeshall RE. Autoradiographic and chemical localization of 5-hydroxytryptamine in identified neurons in the leech. Anat Rec. 1972;172:489–498. doi: 10.1002/ar.1091720303. [DOI] [PubMed] [Google Scholar]
- Coggeshall RE, Lekan HA. Methods for determining numbers of cells and synapses: a case for more uniform standards of review. J Comp Neurol. 1996;364:6–15. doi: 10.1002/(SICI)1096-9861(19960101)364:1<6::AID-CNE2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Cooper RL, Fernandez De Miguel F, Adams WB, Nicholls JG. Anterograde and retrograde effects of synapse formation on calcium currents and neurite outgrowth in cultured leech neurons. Proc R Soc Lond B. 1992;249:217–222. doi: 10.1098/rspb.1992.0107. [DOI] [PubMed] [Google Scholar]
- Davalli AV, Biancardi E, Pollo A, Socci C, Pontiroli AE, Pozza G, Clementi F, Sher E, Carbone E. Dihidropyridine-sensitive and -insensitive voltage-operated Ca2+ channels participate in the control of glucose-induced insulin release from human pancreatic beta cells. J Endocrinol. 1996;150:195–203. doi: 10.1677/joe.0.1500195. [DOI] [PubMed] [Google Scholar]
- Dierkes PW, Hochstrate P, Schlue WR. Voltage-dependent Ca2+ influx into identified leech neurones. Brain Res. 1997;746:285–293. doi: 10.1016/s0006-8993(96)01264-4. [DOI] [PubMed] [Google Scholar]
- Dietzel ID, Drapeau P, Nicholls JG. Voltage dependence of 5-hydroxytryptamine release at a synapse between identified leech neurones in culture. J Physiol. 1986;372:191–205. doi: 10.1113/jphysiol.1986.sp016004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández De Miguel F, Cooper RL, Adams WB. Synaptogenesis and Ca2+ channel distribution in cultured neurons. Proc R Soc Lond B. 1992;247:215–221. [Google Scholar]
- Fernández De Miguel F, Drapeau P. Synapse formation and function: insights from identified leech neurons in culture. J Neurobiol. 1995;27:367–379. doi: 10.1002/neu.480270309. [DOI] [PubMed] [Google Scholar]
- Fuchs PA, Henderson LP, Nicholls JG. Chemical transmission between individual Retzius and sensory neurones of the leech in culture. J Physiol. 1982;323:195–210. doi: 10.1113/jphysiol.1982.sp014068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannucci DR, Yule DI, Stuenkel EL. Optical measurement of stimulus-evoked membrane dynamics in single pancreatic acinar cells. Am J Physiol. 1998;275:C732–739. doi: 10.1152/ajpcell.1998.275.3.C732. [DOI] [PubMed] [Google Scholar]
- Heinemann C, von Rüden L, Chow RH, Neher E. A two-step model of secretion control in neuroendocrine cells. Pflugers Arch. 1993;424:105–112. doi: 10.1007/BF00374600. [DOI] [PubMed] [Google Scholar]
- Henderson L. The role of 5-hydroxytryptamine as a transmitter between identified leech neurones in culture. J Physiol. 1983;339:311–326. doi: 10.1113/jphysiol.1983.sp014718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson LP, Kuffler DP, Nicholls JG, Zhang R. Structural and functional analysis of synaptic transmission between identified leech neurones in culture. J Physiol. 1983;340:347–358. doi: 10.1113/jphysiol.1983.sp014766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang LYM, Neher E. Ca2+-dependent exocytosis in the somata of dorsal root ganglion neurons. Neuron. 1996;17:135–145. doi: 10.1016/s0896-6273(00)80287-1. [DOI] [PubMed] [Google Scholar]
- Jaffe EH, Marty A, Schulte A, Chow RH. Extrasynaptic vesicular transmitter release from the somata of substantia nigra neurons in rat midbrain slices. J Neurosci. 1998;18:3548–3553. doi: 10.1523/JNEUROSCI.18-10-03548.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen J, Kleinhaus AL. Differential sensitivity of tetrodotoxin of nociceptive neurons in 4 species of leeches. J Neurosci. 1986;6:3499–3504. doi: 10.1523/JNEUROSCI.06-12-03499.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilic G, Angleson JK, Cochilla AJ, Nussinovitch I, Betz WJ. Sustained stimulation of exocytosis triggers continuous membrane retrieval in rat pituitary somatotrophs. J Physiol. 2001;532:771–783. doi: 10.1111/j.1469-7793.2001.0771e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristan WB, Jr, Nusbaum MP. The dual role of serotonin in leech swimming. J Physiol (Paris) 1982-83;78:743–747. [PubMed] [Google Scholar]
- Kuffler DP, Nicholls JG, Drapeau P. Transmitter localization and vesicle turnover at a serotoninergic synapse between identified leech neurons in culture. J Comp Neurol. 1987;256:516–526. doi: 10.1002/cne.902560404. [DOI] [PubMed] [Google Scholar]
- Lemmens R, Larsson O, Berggren PO, Islam MS. Ca2+-induced Ca2+ release from the endoplasmic reticulum amplifies the Ca2+ signal mediated by activation of voltage-gated L-type Ca2+ channels in pancreatic beta-cells. J Biol Chem. 2001;276:9971–9977. doi: 10.1074/jbc.M009463200. [DOI] [PubMed] [Google Scholar]
- Lent CM, Dickinson MH. Serotonin integrates the feeding behavior of the medicinal leech. J Comp Physiol A. 1984;154:457–471. [Google Scholar]
- Lockery SR, Kristan Wb., Jr Distributed processing of sensory information in the leech II. Identification of interneurons contributing to the local bending reflex. J Neurosci. 1990;10:1816–1829. doi: 10.1523/JNEUROSCI.10-06-01816.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukyanetz EA, Neher E. Different types of Ca2+ channels and secretion from bovine chromaffin cells. Eur J Neurosci. 1999;11:2865–2873. doi: 10.1046/j.1460-9568.1999.00707.x. [DOI] [PubMed] [Google Scholar]
- McAdoo DJ, Coggeshall RE. Gas chromatographic-mass spectrometric analysis of biogenic amines in identified neurons and tissues of Hirudo medicinalis. J Neurochem. 1976;26:163–167. [PubMed] [Google Scholar]
- Mansvelder HD, Kits KS. Regulation of exocytosis in neuroendocrine cells: spatial organization of channels and vesicles stimulus-secretion coupling Ca2+ buffers and modulation. Prog Neurobiol. 2000;62:427–441. doi: 10.1016/s0165-0173(00)00023-0. [DOI] [PubMed] [Google Scholar]
- Mar A, Drapeau P. Modulation of conduction block in leech mechanosensory neurons. J Neurosci. 1996;16:4335–4343. doi: 10.1523/JNEUROSCI.16-14-04335.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner R, Neher E. The role of calcium in stimulus-secretion coupling in excitable and non-excitable cells. J Exp Biol. 1988;139:329–345. doi: 10.1242/jeb.139.1.329. [DOI] [PubMed] [Google Scholar]
- Puopolo M, Hochstetler SE, Gustincich S, Wightman RM, Raviola E. Extrasynaptic release of dopamine in a retinal neuron: activity dependence and transmitter modulation. Neuron. 2001;30:211–225. doi: 10.1016/s0896-6273(01)00274-4. [DOI] [PubMed] [Google Scholar]
- Regan LJ, San DWY, Bean BP. Ca2+ channels in rat central and peripheral neurons: high-threshold current resistant to dihydropyridine blockers and ω-conotoxin. Neuron. 1991;6:269–280. doi: 10.1016/0896-6273(91)90362-4. [DOI] [PubMed] [Google Scholar]
- Reuter H. Diversity and function of presynaptic calcium channels in the brain. Curr Opin Neurobiol. 1996;6:331–337. doi: 10.1016/s0959-4388(96)80116-4. [DOI] [PubMed] [Google Scholar]
- Sitsapesan R, Williams AJ. Mechanisms of caffeine activation of single calcium-release channels of sheep cardiac sarcoplasmic reticulum. J Physiol. 1990;423:425–439. doi: 10.1113/jphysiol.1990.sp018031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CB, Betz WJ. Simultaneous independent measurement of endocytosis and exocytosis. Nature. 1996;380:531–534. doi: 10.1038/380531a0. [DOI] [PubMed] [Google Scholar]
- Stewart RR, Adams WB, Nicholls JG. Presynaptic calcium currents and facilitation of serotonin release at synapses between cultured leech neurones. J Exp Biol. 1989;144:1–12. doi: 10.1242/jeb.144.1.1. [DOI] [PubMed] [Google Scholar]
- Steyer JA, Almers W. Tracking single secretory granules in live chromaffin cells by evanescent-field fluorescence microscopy. Biophys J. 1999;76:2262–2271. doi: 10.1016/S0006-3495(99)77382-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P, Surprenant A, Almers W. Cytosolic Ca2+ exocytosis and endocytosis in single melanotrophs of the rat pituitary. Neuron. 1990;5:723–733. doi: 10.1016/0896-6273(90)90226-6. [DOI] [PubMed] [Google Scholar]
- Triggle DJ, Janis RA. Calcium channel ligands. Ann Rev Pharmacol Toxicol. 1987;27:347–369. doi: 10.1146/annurev.pa.27.040187.002023. [DOI] [PubMed] [Google Scholar]
- Usachev YM, Shmigol A, Pronchuk N, Kostyuk P, Verkhratsky A. Caffeine-induced calcium release from internal stores in cultured rat sensory neurons. Neuroscience. 1993;57:845–859. doi: 10.1016/0306-4522(93)90029-f. [DOI] [PubMed] [Google Scholar]
- Villalobos C, Nuñez L, García-Sancho J. Mechanisms for stimulation of rat anterior pituitary cells by arginine and other amino acids. J Physiol. 1997;502:421–431. doi: 10.1111/j.1469-7793.1997.421bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willard AL. Effects of serotonin on the generation of the motor program for swimming by the medicinal leech. J Neurosci. 1981;1:936–944. doi: 10.1523/JNEUROSCI.01-09-00936.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RJ, Kristan Wb, Jr, Kleinhaus AL. An increase in activity of serotonergic Retzius neurons may not be necessary for the consummatory phase of feeding in the leech Hirudo medicinalis. J Exp Biol. 1996;199:1405–1414. doi: 10.1242/jeb.199.6.1405. [DOI] [PubMed] [Google Scholar]
- Yaksta-Sauerland BA, Coggeshall RE. Neuromuscular junctions in the leech. J Comp Neurol. 1973;151:85–99. doi: 10.1002/cne.901510107. [DOI] [PubMed] [Google Scholar]
- Zaidi ZF, Matthews MR. Exocytotic release from neuronal cell bodies dendrites and nerve terminals in sympathetic ganglia of the rat and its differential regulation. Neuroscience. 1997;80:861–891. doi: 10.1016/s0306-4522(96)00664-1. [DOI] [PubMed] [Google Scholar]
- Zaidi ZF, Matthews MR. Stimulant-induced exocytosis from neuronal somata dendrites and newly formed synaptic nerve terminals in chronically decentralized sympathetic ganglia of the rat. J Comp Neurol. 1999;415:121–143. doi: 10.1002/(sici)1096-9861(19991206)415:1<121::aid-cne9>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Zorec R, Sikdar SK, Mason WT. Increased cytosolic calcium stimulates exocytosis in bovine lactotrophs. Direct evidence from changes in membrane capacitance. J Gen Physiol. 1991;97:473–497. doi: 10.1085/jgp.97.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]


