Figure 5. [Ca2+]i elevations and Cl− currents in response to GABA and glycine.
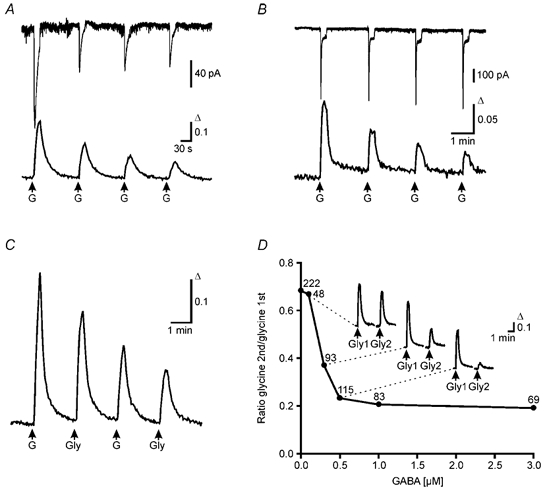
A, combined gramicidin perforated-patch recording and calcium imaging at DIV 8. Amplitude of the Cl− current of a single cell obtained by perforated-patch recording (top trace, VH = −60 mV) and the simultaneously recorded Ca2+ transients of the whole cell population (bottom trace, average of 32 cells) declined exponentially with repetitive application of GABA (50 μm, 15 s pulse). B, combined whole-cell patch recording and calcium imaging at DIV 13. Amplitude of current responses from a whole-cell patch recording (top trace, VH = −75 mV) remained constant during repetitive application of GABA (50 μm, 15 s pulse). In contrast the Ca2+ transients recorded in parallel (bottom trace, average of 15 cells) declined. C, average of Ca2+ transients elicited by alternating GABA and glycine applications (50 μm and 500 μm, respectively, 15 s pulse, 12 cells) from a culture at DIV 7. D, nanomolar concentrations of GABA suffice to reduce glycine-evoked Ca2+ transients. Between two glycine applications, BPTTX-incubated cultures (DIV 9–13) were superfused for 20 min with low GABA concentrations (0.1–3 μm). The ratios between the amplitudes of the second (Gly2) and the first (Gly1) glycine responses were plotted against the GABA concentration. Insets show averaged responses for control (n = 17), 0.3 μm GABA (n = 35) and 0.5 μm GABA (n = 23). Number of cells indicated for each point.
