Figure 9. sgk1 associates with the membrane fraction of kidney homogenates.
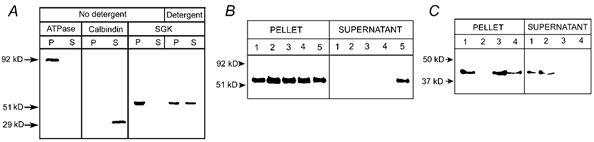
A, kidneys from normal rats were homogenized in the absence of detergent or in the presence of 1 % Triton X-100. The homogenates were centrifuged at 250 000 g for 30 min and equal amounts of proteins from pellet and supernatant were examined by Western blotting with antibodies for the α subunit of Na+,K+-ATPase, calbindin and sgk1. Na+,K+-ATPase (ATPase) and sgk1 were detected only in the pellet of non-detergent homogenates whereas calbindin was detected in the supernatant. After treatment of the pellet with 1 % Triton X-100, sgk1 was partially released to the supernatant. B, the pellet obtained after the first centrifugation (250 000 g for 30 min) was resuspended in 200 μl of: (1) 0.3 m sucrose, (2) 0.1 m Na2CO3, pH 11.5, (3) 0.5 m NaCl, (4) 2 m urea or (5) 1 % Triton X-100, and centrifuged again at 250 000 g for 30 min. Equal amounts of proteins from the pellets and 30 μl from the supernatant were loaded on a gel and analysed by Western blotting with anti-sgk1 antibody. C, Western blot similar to that shown in B, but probed with a monoclonal anti-actin antibody. Arrows indicate migration of molecular mass markers.
