Abstract
We have examined the regulation of lipoprotein lipase (LPL) activity in skeletal muscle during physical inactivity in comparison to low-intensity contractile activity of ambulatory controls. From studies acutely preventing ambulatory activity of one or both the hindlimbs in rats, it was shown that ≈90–95 % of the heparin-releasable (HR) LPL activity normally present in rat muscle with ambulatory activity is lost, and thus dependent on local contractile activity. Similarly, ≈95 % of the differences in LPL activity between muscles of different fibre types was dependent on ambulatory activity. The robustness of the finding that physical inactivity significantly decreases muscle LPL activity was evident from confirmatory studies with different models of inactivity, in many rats and mice, both sexes, three muscle types and during both acute and chronic (11 days) treatment. Inactivity caused a local reduction of plasma [3H]triglyceride uptake into muscle and a decrease in high density lipoprotein cholesterol concentration. LPL mRNA was not differentially expressed between ambulatory controls and either the acutely or chronically inactive groups. Instead, the process involved a rapid loss of the HR-LPL protein mass (the portion of LPL largely associated with the vascular endothelium) by an actinomycin D-sensitive signalling mechanism (i.e. transcriptionally dependent process). Significant decreases of intracellular LPL protein content lagged behind the loss of HR-LPL protein. Treadmill walking raised LPL activity ≈8-fold (P < 0.01) within 4 h after inactivity. The striking sensitivity of muscle LPL to inactivity and low-intensity contractile activity may provide one piece of the puzzle for why inactivity is a risk factor for metabolic diseases and why even non-vigorous activity provides marked protection against disorders involving poor lipid metabolism.
One of the most important environmental changes influencing human physiology and disease in recent years has been the decline of physical activity that was a routine part of most people's lives. The typical person in hunter-gatherer and agrarian societies spent almost the entire day performing low-intensity muscular work or ambulatory activity. Epidemiological research over the past 50 years has conclusively and repeatedly demonstrated that inactivity is a major risk factor for death, primarily due to increased coronary heart disease (CHD) (Morris et al. 1953; Haskell et al. 1994). Other disorders involving poor skeletal muscle lipid metabolism are now on the rise (syndrome X, type II diabetes, obesity). Epidemiological research has often focused on the increased risk of disease in the most physically inactive people. Although somewhat controversial, the general consensus is that the dose-response relationship between activity and disease is steep, i.e. the greatest benefit would come from getting the least active to become moderately active (Haskell et al. 1994; Blair & Brodney, 1999; Kesaniemi et al. 2001). There has been a paradigm shift in the recommendations from consensus panels emphasizing the importance of avoiding the ill effects of physical inactivity by frequently incorporating moderate activity into the daily routine, and not just the more intense types of formalized endurance exercise training. Thus, there is a growing need to understand the underlying processes most sensitive to inactivity and low-intensity activity.
LPL has been studied heavily because this enzyme has a central role in several aspects of lipid metabolism (Olivecrona et al. 1997; Goldberg & Merkel, 2001). LPL also has a major influence on the partitioning of triglyceride-derived fatty acid uptake between different tissues, plasma cholesterol metabolism and the subsequent downstream intracellular effects related to lipid availability. Most dramatically, a partial reduction in LPL function because of a specific polymorphism was associated with a 5-fold increase in the odds ratio for death and greater CHD (Wittrup et al. 1999). Studies using transgenic overexpression (Levak-Frank et al. 1997), intramuscular LPL DNA injection (Schlaepfer & Eckel, 1999), acute administration of LPL antibodies (Goldberg et al. 1988), gene knockout technology (Levak-Frank et al. 1997) or LPL-altering drugs have all led to the conclusion that LPL is critical for the tissue-specific uptake of triglyceride-rich lipoproteins by non-hepatic tissues. In a study of a cohort of 730 patients, those with documented CHD had less post-heparin LPL activity compared with healthy controls (Henderson et al. 1999), and a smaller study observed that the clearance of chylomicron-like triglyceride emulsions was delayed (Martins et al. 1995). Thus, compelling arguments have recently been made that the physiological modulation of LPL activity may contribute to the aetiology or prevention of the metabolic disorders.
Owing to the wide swings in the local metabolic demands of skeletal muscle, understanding the biochemical plasticity in this tissue has been of high interest in medical research (Hamilton & Booth, 2000). Most of the emphasis on mechanisms regulating muscle LPL has concerned high-intensity exercise. Less is known about the contrast of inactivity to low-intensity contractions. If the underlying mechanisms regulating LPL happen to be dependent on intensity, then there would be much to be gained by insights about lower intensities that are more safe and feasible to perform, especially by the obese and/or elderly. For example, ageing reduces LPL activity (Bey et al. 2001; Hamilton et al. 2001) and the very sedentary elderly are encouraged to walk moderately (Frandin et al. 1991). Thus, we sought to understand why regulation of LPL activity in muscles may be different during inactivity compared with muscles contracting in the low-intensity range of the physical activity spectrum in either control animals or animals walking slowly on a treadmill after inactivity. We tested the hypothesis that the normally high LPL activity in muscle (especially in oxidative muscle) is significantly decreased by physical inactivity compared with ambulatory controls and that restoring ambulation in previously inactive animals would raise muscle LPL activity. Insights regarding the responsible mechanisms were obtained from measurements of LPL activity and protein mass in the functionally important heparin-releasable fraction and the larger intracellular precursor pool during physical inactivity or low-intensity activity, as well as determining whether such changes involve differential expression of LPL mRNA. The study design also allowed determination of whether local stimuli associated with contractile activity are responsible for muscle LPL regulation, and made a comparison of fibre types. Completing these aims could give a new perspective about the physiological processes for how LPL is regulated during an important cause for metabolic disorders, namely physical inactivity.
METHODS
Animal protocols
Female (n = 166) and male (n = 12) Sprague-Dawley rats (Harlan), weighing ≈175–220 g and 350–420 g, respectively, were maintained in a temperature- and light-controlled environment (12 h:12 h light-dark cycle) and fed standard rat chow diet. Food was removed 5–6 h prior to experiments to control for potentially variable rates of food intake and possible uncontrolled interactive effects of treatment. Female C57BL/6 mice (Harlan; n = 12) weighing ≈18 g were treated similarly. Animal procedures were approved by the University of Missouri Animal Care and Use Committee and performed in respect of American Physiology Society principles for research on animals.
Hindlimb unloading (HU) was performed to prevent weight-bearing activity in the hindlimbs by wrapping 1.5 cm of the tail with adhesive tape connected to a fishing lure swivel tied to an overhanging metal rod. The hindlimbs were elevated just enough to prevent the rear feet from touching the floor, thus providing a model to reduce contractile activity localized primarily to the two hindlegs. All animals had unrestricted access to move about in the cage with forelimbs for essential activities. Rats were accustomed to HU for 1–2 h day−1 for several days. HU was initiated at the beginning of the dark cycle.
A subset of rats (n = 4) was injected intraperitoneally (I.P.) with 2 mg kg−1 actinomycin D (Sigma) prior to 6 h HU and compared with vehicle (0.4 ml of 40 % ethanol-saline solution) or actinomycin control groups (n = 4–8).
In a chronic study, eight rats were inactive with HU for 10 h day−1 for 11 days and compared with control rats without HU. In an acute study, normal cage activity and low-intensity ambulatory activity was re-established following 12 h of HU (n = 20) with a 4 h reloading phase. This 4 h reloading phase consisted of returning the rats to normal cage activity and quadripedal locomotion involving treadmill walking at a low speed (8 m min−1) for 30 min each hour (four intermittent bouts in 4 h). Control rats (n = 6) were treated similarly during this 4 h period. Two additional groups of five 12 h hindlimb unloaded rats were also reloaded for 4 h after injection I.P. with 2 mg kg−1 actinomycin D or vehicle. One group of rats (n = 6) had a single cut of the soleus tendon and was studied 12 h later after fully recovering from anaesthesia (a mixture of ketamine (54 mg ml−1), xylazine (2.2 mg ml−1) and acepromazine (3.5 mg ml−1) given in a volume of 1.4 ml kg−1 I.P.).
Post-heparin eluate and tissue preparation
Muscles were removed at the end of the HU or the reloading phase for all groups after anaesthetizing the animals as described above. All animals were killed by removing the heart. Heparin-releasable LPL (HR-LPL) was obtained from non-frozen tissue (≈30–50 mg) minced to < 10 mg pieces and soaked immediately in PBS buffer containing 20 U ml−1 heparin (Sigma) for 30 min at 37 °C. The eluate was stored at −80 °C.
Total LPL activity in muscle samples was determined from homogenates in a Tris buffer containing heparin and several protease inhibitors as described previously (Hamilton et al. 1998). Residual LPL activity was calculated after subtraction of HR from total LPL activity. For total LPL protein, muscle was homogenized in a Tris buffer containing 1 % Triton X-100, 0.1 % SDS, 0.5 % deoxycholate, 5 U ml−1 heparin and several protease inhibitors, and centrifuged at 1751 g for 10 min at 4 °C to remove tissue debris. Total protein concentration in the supernatant was measured using a Bio-Rad Dc protein assay kit to load equal amounts of protein onto gels.
LPL enzyme activity
Muscle homogenates and post-heparin eluates were assayed for LPL enzyme activity using a [3H]triolein substrate as described previously (Hamilton et al. 1998). Each sample was measured in duplicate and at two different concentrations to ensure reproducibility and linearity. LPL activity is reported in units of nanomoles of fatty acids hydrolysed per gram of muscle per minute and also as the percentage difference from ambulatory active controls.
LPL protein mass
LPL protein mass in the muscle supernatants and post-heparin eluates was determined using a chicken polyclonal anti-bovine LPL antibody (gift from Dr John Goers) as described previously (Bey et al. 2001). Total protein was separated by 7 % SDS-PAGE and transferred to PVDF nitrocellulose membrane (Millipore). The membrane was blocked for 1 h with 5 % bovine serum albumin in Tris buffer and hybridized for 2 h with 1:5000 anti-bovine LPL antibody followed by 1:100 000 anti-chicken antibody conjugated with horseradish peroxidase for 1 h. The membrane was developed with the enhanced chemical luminescence-plus kit (Amersham Pharmacia Biotech Inc.). The integrated optical density of each band was quantified from the autoradiogram.
LPL mRNA concentration
Quantitative Northern blot analysis was performed with total RNA extracted using methods described in detail and illustrated previously (Hamilton et al. 1998).
Plasma triglycerides (TGs) and high density lipoprotein cholesterol (HDL-C)
Plasma was prepared from blood collected at the femoral artery. Plasma TG concentration was measured using Infinity Triglycerides Reagent (Sigma). The HDL fraction in plasma was separated from the LDL fraction using HDL Cholesterol Reagent (PTA/MgCl2; Sigma) and cholesterol concentration in this HDL fraction was determined using Infinity Cholesterol Reagent (Sigma).
[3H]TG uptake
TG uptake by skeletal muscle and heart was determined from the accumulation of [3H]TG-derived fatty acids as an in vivo index of LPL activity in regional vascular beds (Linder et al. 1976; Augustus et al. 2003). [3H]Triolein (600 μCi; Perkin-Elmer) and 300 mg unlabelled triolein (Sigma) were emulsified as a purified source of TG with 18 mg phosphatidyl choline (Sigma). The lipid was injected as a bolus into a central catheter at a dosage of 14 × 106 c.p.m. (8.87 mg TG in a volume of 200 μl) in six rats weighing ≈220 g. Ten minutes after injection of tracer, the red quadriceps and heart were collected as described above for subsequent lipid extraction. After weighing and powdering ≈100 mg of the tissues, each was homogenized in chloroform:methanol (2:1). The mixture was completely evaporated following centrifugation at 875 g for 15 min. Duplicates of each tissue sample and pellet were counted in scintillation fluid. Results showed that at least 90 % of the radiation in each tissue was extracted in the supernatant.
Statistics
All results are reported as means ± s.e.m. The significance was calculated with ANOVA, and followed by Tukey-Kramer tests when there were multiple comparisons. Results were considered significant at P < 0.05.
RESULTS
Muscle weight is not altered by intermittent inactivity
An intermittent pattern of inactivity and activity (10 h day−1 of HU) was studied after 11 days. In contrast to some of the other rodent models of continuous inactivity that cause a large muscle atrophy after several days, the soleus muscle did not atrophy in the present study (175 ± 4.4 vs. 175 ± 4.3 mg in control and HU, respectively).
LPL activity is profoundly reduced by physical inactivity in skeletal muscles compared with low-intensity ambulatory activity
Eleven days of inactivity significantly reduced HR-LPL activity in skeletal muscle (Fig. 1A). This large decrease in muscle LPL was not caused by an accumulative effect each of those 11 days, because there was not a statistical difference between a single day of inactivity and 11 days of inactivity (Fig. 1A). In order to elucidate the time course for the changes in muscle LPL in this model of inactivity, we next studied the soleus (representative of slow twitch oxidative fibres) and red quadriceps (RQ) muscle section (mixed with a large contribution of fast twitch oxidative fibres) during the first 18 h of HU (n = 3–8 rats per group). After a 4 h lag period, both HR-LPL and intracellular LPL activities decreased mono-exponentially in the soleus (Fig. 1B, C and D) and RQ muscles (Fig. 1D). The maximal steady- state decrease reached at < 18 h was to 6 % of control (Fig. 1B). During the decrease, the half-life of LPL activity in both muscle types was ≈2 h (Fig. 1D). Male and female rats had a similar LPL response to HU (Table 1). Mice also showed a 62–64 % loss of both HR-LPL and total LPL activity (n = 6, P < 0.01) in their soleus muscles after 12 h HU (data not illustrated).
Figure 1. Time course for the decrease in muscle LPL activity after inactivity (hindlimb unloading, HU) compared with low-intensity ambulatory activity.
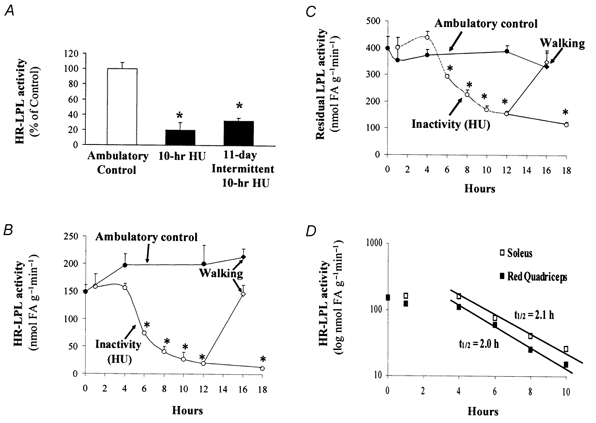
Soleus HR-LPL activity was decreased after acute (1 day for 10 h, n = 3) or chronic (11 days of 10 h day−1, n = 8) HU (A). The time course of HR-LPL (B) and intracellular LPL (C) activities in soleus muscle within the first 18 h of HU revealed a delay of ≈4 h and then a precipitous decrease (n = 3–5). Restoring contractile activity for 4 h reversed the decrease in muscle LPL induced by 12 h inactivity. Control rats with normal cage activity (n = 5) and 12 h hindlimb unloaded rats (n = 20) were subjected to 4 h of intermittent treadmill walking (30 min intervals at 8 m min−1 four times separated by 30 min of ‘free in the cage’) and compared with rats with normal ambulatory activity or after 12 h HU. A direct comparison of the time course in the red oxidative slow twitch (soleus) and fast twitch (red quadriceps, RQ) muscles is shown (D). The half-life of LPL activity was calculated from 4 to 10 h using t1/2 = ln 2/k, where k = −1n (A(t)/A(0))/t. The average k calculated from 4 to 10 h was 0.334 for soleus and 0.341 for RQ. *P < 0.01 between HU and control.
Table 1.
HR-LPL activity in skeletal muscle of both female and male rats during inactivity
| Soleus (nmol FA g−1 min−1) | Red quadriceps (nmol FA g−1 min−1) | |
|---|---|---|
| Male | ||
| Ambulatory control | 200 ± 30 | 178 ± 23 |
| 4 h HU | 183 ± 14 | 138 ± 26 |
| 18 h HU | 16 ± 0.3* | 24 ± 1.8* |
| Female | ||
| Ambulatory control | 197 ± 21 | 177 ± 11 |
| 4 h HU | 156 ± 8 | 110 ± 16* |
| 18 h HU | 11 ± 1.2* | 21 ± 4.8* |
P < 0.01 between HU (n = 3–4) and ambulatory control (n = 4–5), HU, hindlimb unloading; FA, fatty acid.
Restoring contractile activity for 4 h is sufficient to reverse the decrease in muscle LPL activity induced by inactivity
Both soleus HR-LPL and residual LPL activity returned to control levels when 12 h hindlimb unloaded rats were reloaded with 4 h standing and slow walking on a treadmill (Fig. 1B and C). Similarly, the decrease in HR-LPL activity in the RQ muscles after 12 h HU was also reversed to control levels after 4 h reloading (100 ± 14 vs. 80 ± 12 % in treadmill-walked control (n = 5) and reloaded HU (n = 20), respectively).
Comparison of muscle fibre types
We next extended the findings shown in Fig. 1 to many more animals (n = 35) with 12 h inactivity and observed in every case a robust reduction of LPL activity. HR-LPL activity was 10-fold less (P < 0.01) in both soleus (n = 35) and RQ (n = 7) muscles from 12 h hindlimb unloaded rats compared with low-intensity ambulatory controls (Fig. 2). HR-LPL activity in the rectus femoris (RF) muscle, a largely white glycolytic fast twitch muscle, was also about 2-fold less (P < 0.05) in the inactive rats than in the more active controls (Fig. 2).
Figure 2. LPL activity in different muscle fibre types after 12 h inactivity compared with low-intensity ambulatory activity.
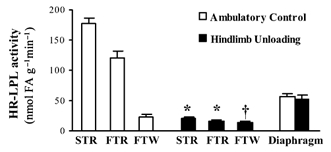
HR-LPL activity was determined in a slow twitch red (STR) soleus (n = 35), fast twitch red (FTR) deep quadriceps (n = 7) and fast twitch white (FTW) or superficial white region of rectus femoris (n = 6) and the diaphragm, which is a mixed skeletal muscle with continual activity (n = 7). *P < 0.01, †P < 0.05 between HU and low-intensity ambulatory control. LPL activity between all three hindlimb muscles was significantly different in controls but not after HU removed the normal contractile activity.
A local process is responsible for the loss of muscle LPL activity during physical inactivity
The lack of a decrease in LPL activity for the heart (100 ± 11 % and 115 ± 4 % in control and HU, respectively) and diaphragm (Fig. 2), two muscles with high oxidative capacity and LPL activity but continual contractile activity, revealed that the loss of muscle LPL was restricted locally to the hindlimbs during HU. Tenotomy of the soleus tendon was then employed to test the hypothesis that unilateral unloading of one hindlimb would only decrease muscle LPL activity in the unloaded limb compared with the loaded limb. We noticed that the rat was not weight-bearing on the leg with soleus tenotomy and thus studied the RQ muscle as an unloaded but non-tenotomized muscle. HR-LPL activity in the RQ of the unloaded hindlimb was decreased to 20 % of the control group and 18 % of the paired contralateral limb (both P < 0.01; Table 2). Minimizing local contractile activity also reduced soleus HR-LPL activity as expected (Table 2). The lack of significant effects in contralateral hindlimb muscles or diaphragm also supported the interpretation that a local process is responsible for the effect of physical inactivity.
Table 2.
HR-LPL activity in skeletal muscle after unilateral hindlimb inactivity
| Soleus (% of control) | Red quadriceps (% of control) | Diaphragm (% of control) | |
|---|---|---|---|
| Ambulatory control | 100 ± 12 | 100 ± 16 | 100 ± 15 |
| Contralateral leg | 85 ± 9 | 108 ± 15 | — |
| 12 h after tenotomy to Achilles tendon‡ | 6 ± 2*† | 20 ± 4*† | 80 ± 18 |
There was tenotomy of the soleus tendon but no tenotomy of the red quadriceps (RQ) tendon. RQ was obtained from the most deep and red region of the three vastus muscles. The foot of the one leg with tenotomy was not weight-bearing and thus the RQ muscle was unloaded in that leg, and this RQ muscle provided and oppurtunity to study unilateral unloading. Tenotomy was performed on one hindlimb by cutting the distal soleus tendon (n = 6).
P < 0.01 between unloaded and control muscles,
P < 0.01 between paired unloaded and loaded contralateral muscles in the same rats.
Inactivity reduces TG uptake by hindlimb muscles and plasma HDL-C
It has been well established that tissue-specific TG uptake is determined primarily by the amount of LPL activity present locally in tissues (Linder et al. 1976; Mackie et al. 1980; Levak-Frank et al. 1997; Goldberg & Merkel, 2001; Augustus et al. 2003) and TG uptake in muscle can change quickly during acute electrical stimulation compared with resting muscle in anaesthetized rats (Mackie et al. 1980). Consistent with this, there was a significant decrease in [3H]TG-derived fatty acid uptake and HR-LPL during inactivity locally in hindlimb skeletal muscle, but no decrease in either measure in the heart (Fig. 3). The fasting plasma TG concentration was not significantly affected by HU in these animals (36 ± 2.8 vs. 43 ± 2.6 mg dl−1 in HU and control (n = 7), respectively). HDL-C concentration was significantly reduced after both 1 and 11 days (Fig. 3).
Figure 3. Inactivity decreases both muscle TG uptake and plasma HDL-C.
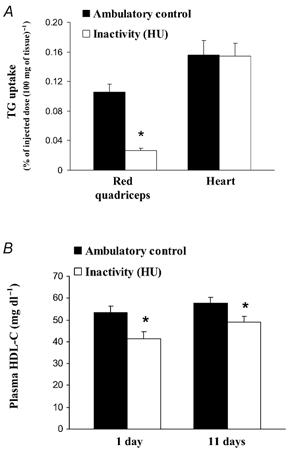
The accumulation of TG-derived fatty acids in the RQ hindlimb muscles and heart (n = 3) was determined 10 min after injection of [3H]triolein-labelled chylomicron-like emulsion in rats following 18 h of HU and compared with rats with normal ambulatory activity (A). Rats with both 1 day (n = 6) and 11 days (n = 8) of intermittent (10–12 h HU day−1) inactivity had a significantly lower plasma HDL-C concentration compared with active controls (B). *P < 0.05 between HU and low-intensity ambulatory control.
LPL mRNA and protein
In spite of the large decrease in LPL activity in the soleus muscle during 12 h HU, there was not a significant change in LPL mRNA concentration using Northern blot (n = 12; Table 3) or, in a recently published work, real-time quantitative RT-PCR (100 ± 21 vs. 137 ± 7 % (n = 6) in control and HU, respectively) with another set of animals (Bey et al. 2003). Furthermore, 11 days of 10 h intermittent HU (n = 8) did not cause a significant change in LPL mRNA concentration (Table 3). LPL mRNA concentration decreased to 27 % of control (P < 0.01) after 24 h day−1 of HU for 7 days in the soleus muscle (Table 3). LPL mRNA concentration in the RF muscle remained unchanged after 12 h or 7 days (24 h day−1) HU (Table 3). Both HR and total LPL protein mass were significantly decreased (P < 0.01) after 12 h HU (Table 4). Although both total and HR-LPL activities were already decreased after 6 h HU, only the HR-LPL protein mass was decreased at this early time (Table 4).
Table 3.
LPL mRNA concentration during acute and chronic inactivity
| Soleus (% of control) | Rectus femoris (% of control) | |
|---|---|---|
| Ambulatory control (n = 4–12)‡ | 100 ± 6 | 100 ± 4 |
| 12 h HU (n = 12) | 91 ± 6 | 87 ± 14 |
| 11 days of 10 h day−1 HU (n = 8) | 69 ± 9 | — |
| 7 days of 24 h day−1 HU (n = 4) | 27 ± 8*† | 101 ± 9 |
HU groups were compared with respective controls studied at the same time (n = 12 for 12 h and n = 4–8 for 7–11 days).
P < 0.01 between HU and control,
P < 0.01 between intermittent and continuous HU.
Table 4.
HR-LPL and total LPL protein mass in skeletal muscle after inactivity
| HR-LPL protein (% of control) | Total LPL protein (% of control) | |
|---|---|---|
| Ambulatory control | 100 ± 7 | 100 ± 13 |
| 4 h HU | 124 ± 30 | 108 ± 16 |
| 6 h HU | 59 ± 5* | 106 ± 30 |
| 12 h HU | 12 ± 5* | 54 ± 6* |
All values are for soleus muscle.
P < 0.01 between HU and ambulatory control. n = 6 for both control and HU at 12 h and n = 3–4 for other durations.
A transcriptionally mediated event signalled for the changes in the regulation of muscle LPL during inactivity
Actinomycin D (ActD) is a potent global inhibitor of transcription, and has been shown to raise LPL activity in adipose tissue without raising LPL mRNA concentration (Shotz & Garfinkel, 1965; Bergo et al. 2002). We tested the hypothesis that there may also be a signalling pathway in the inactive skeletal muscle that requires the ongoing transcription of a short-lived and potent ‘LPL suppressor gene’, and thus ActD may prevent the rapid decrease in LPL activity during inactivity. In three groups of rats with 6 h HU, the average decrease for the soleus (n = 11) and RQ (n = 7) HR-LPL activities was 51 and 40 % of control level, respectively (Table 5). Intraperitoneal injection of ActD into rats immediately prior to hindlimb suspension completely prevented this decrease in HR-LPL activity in both muscle types (Table 5). Interestingly, ActD did not change LPL activity in ambulatory control animals (Table 5). Also, injection of ActD at the beginning of 4 h of restoring contractile activity in 12 h hindlimb unloaded rats did not alter the rise of muscle LPL activity induced by treadmill walking (100 ± 3 % in reloaded HU vs. 108 ± 13 % in reloaded HU + ActD; n = 5).
Table 5.
The inhibition of transcription prevents the decrease of HR-LPL activity induced in hindlimb muscl during inactivity
| Soleus (% of control) | Red quadriceps (% of control) | |
|---|---|---|
| Ambulatory control (n = 4) | 100 ± 9 | 100 ± 13 |
| Ambulatory control + ActD (n = 4) | 109 ± 2 | 103 ± 8 |
| 6 h HU (n = 7–11)‡ | 51 ± 6* | 40 ± 6* |
| 6 h HU + ActD (n = 4) | 126 ± 14ns | 105 ± 8ns |
HR-LPL activity after 6 h HU alone was obtained from three independent groups for soleus (n = 11) and two for RQ (n = 7) and comared with respective controls killed at the same time (n = 12). ns, no statistical difference was observed between HU and control after the inhibition of transcription using one-way ANOVA,
P <0.01 between HU and ambulatory control.
DISCUSSION
Compelling arguments by others have pointed towards an optimal balance of tissue-specific LPL activity as a molecular target for common lipid disorders, and high LPL in muscle has been suggested to be more related to favourable lipid metabolism than high adipose LPL. This study is relatively unique from many of the previous studies on muscle LPL because it has sought to understand the steps of muscle LPL regulation associated with physical inactivity and low-intensity contractions. These novel insights on this relationship may be of use because it is now clear that alterations of key molecular steps for muscle LPL regulation that are sensitive to inactivity can be prevented and reversed by even the non-fatiguing contractions that are relatively easy and safe to perform, and thus may provide an additional reason to support the public health message based largely upon epidemiology (Blair & Brodney, 1999; Kesaniemi et al. 2001). We found that almost all (up to 95 %) of the endothelial-enriched HR-LPL activity normally present in the capillaries of muscles was dependent upon low-intensity ambulatory activity (Fig. 1, Table 2). The conclusion that muscle LPL activity decreases during inactivity and is very sensitive to contractions was based upon a robust set of data. That is: (1) a rapid loss of LPL activity was replicated in several independent groups of rats; (2) LPL activity decreased in the three hindlimb muscle types examined; (3) LPL activity was rapidly reversed by slow treadmill walking; (4) the loss of LPL activity was observed in both sexes and also in mice; (5) changes in LPL activity were verified with different assays for total homogenates and heparin eluates; (6) LPL activity results were in agreement with independent measurements of TG-derived fatty acid uptake in vivo; and (7) two different methods of inactivity were used and similar results were obtained. The effect was not cumulative because there was no statistical difference in the magnitude of the decrease in muscle LPL activity after 1 or 11 days of reduced activity (Fig. 1). Thus, the study includes the most complete information available on the effect of inactivity on LPL.
The large difference in LPL activity between inactive and active muscles after a short period of time adds insights into why LPL is often reported to be different between fibre types and possibly the differences between inactive and active people. It has long been known that LPL activity is generally severalfold greater in the red oxidative muscle types than in white glycolytic muscles in ambulatory control rats (Hamilton et al. 1998; Ladu et al. 1991). However, now we can see (Fig. 2) that removing the normally high level of postural support by oxidative muscles was sufficient to abolish the difference of LPL activity between muscle fibre types. This suggests that the difference in LPL activity between fibre types is primarily due to the level of recruitment in normal daily activity rather than intrinsic factors such as cellular lineage or neurotrophic factors. About a 7-fold range in muscle LPL activity has been shown several times before between young people, and Lithell et al. (1981b) suggested that this was related to capillary density. However, now we know that such large differences in HR-LPL between people (Herd et al. 2001) and rat muscles (present study) are more transient than capillarization or morphological features. Close inspection of individual responses to acute moderate activity has also alluded to the potential for muscle LPL activity to rise and fall in some people by the same striking magnitude as we reported (Lithell et al. 1981a; Herd et al. 2001). Thus it is now clear that local changes in metabolism during even moderate contractions are the most important physiological stimulus for LPL regulation in skeletal muscle.
A balanced appraisal of the existing LPL research shows that many, but not all, of the studies provide evidence that LPL is responsive to altered physical activity. A thorough enough picture is emerging about muscle LPL regulation to learn physiological lessons from even the negative results. Take for example the seemingly straightforward question, does prolonged walking acutely raise skeletal muscle LPL activity? Even well performed studies could lead to potentially erroneous conclusions about the sensitivity of muscle LPL to moderate intensity activity incorporated as part of the daily routine. The acute treadmill walking data are an example. There was a dramatic increase in HR-LPL activity in the oxidative muscles (soleus and RQ) of animals during walking that had been previously sedentary (Fig. 1). In contrast, there was absolutely no trend for an increase in these two oxidative muscles when LPL was already high from normal cage activity (Fig. 1B and C, and Fig. 4 upper panel). Furthermore, this null result at 8 m min−1 was not because of a sub-threshold exercise intensity, because prior work (Hamilton et al. 1998) in the two oxidative muscle types showed that running at a seven times faster speed for 3–4 h day−1 also had no effect on oxidative muscles with already high LPL (Fig. 4). It is also apparent that a true assessment of the magnitude of the change in regulation of LPL activity and protein in the hours following moderate exercise or at the onset of inactivity requires careful analysis of the complex temporal patterns of muscle metabolism.
Figure 4. Summary of the dose-response relationship between physical activity/inactivity and muscle LPL.
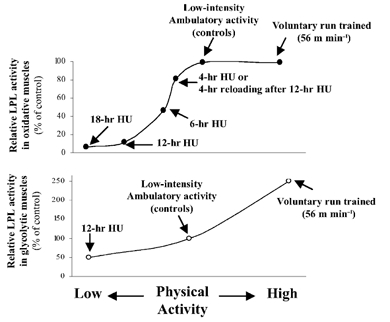
Upper panel, summary of oxidative muscle sections (deep RQ and soleus); lower panel, summary of more glycolytic muscle sections (superficial white vastus lateralis and RF). Results for each muscle type are normalized to ambulatory control values. Absolute values for controls are shown in Fig. 2. Treadmill walking was at 8 m min−1. The data on the effects of low-intensity physical activity are from the current results, and those on high-intensity running (56 m min−1 3.5 h day−1) are from published work (Hamilton et al. 1998). The two studies used the same strain and vendor of rats.
Figure 4 suggests that there is not a ‘minimal intensity threshold’ for the benefits on LPL regulation, but this could depend on the fibre type examined or the point at which ‘control activity’ is defined. Furthermore, fast twitch white muscle fibres have a greater recruitment threshold and are unlikely to be used much in normal ambulatory activities (Hennig & Lomo, 1985), and also generally have low LPL activity and protein unless there is exposure to intense exercise (Fig. 4).
There are several reasons to believe that the local LPL reductions in the oxidative hindlimb muscles were secondary to a reduction in the local energy demand, and that a more generalized hormonal signal was not sufficient to explain the results. Using a model of unilateral unloading, we found that only muscles of the unloaded hindlimb had a decrease in LPL activity (Table 2). In contrast, neither of the same oxidative muscles in the contralateral loaded hindlimb or other non-hindlimb oxidative muscles had a change in LPL activity (Table 2). Smol and colleagues (2001) made the important observation that adding denervation to a tenotomized soleus muscle did not produce an added effect on the loss of LPL activity. HU decreases the load to a muscle and also causes a local reduction of EMG activity during the first 24 h (Alford et al. 1987) or longer (Riley et al. 1990). A reduced energy demand by HU muscle is also evidenced by the rapid and sustained decrease in the local blood flow rate (McDonald et al. 1992; Colleran et al. 2000), especially evident in the oxidative muscles with frequent recruitment during normal postural and ambulatory activities (Hennig et al. 1985). The decrease in LPL in the hindlimb was larger in the oxidative slow and fast twitch red muscles compared with glycolytic fast twitch white muscles (Fig. 2), probably because the oxidative muscles had the greatest LPL and contractile activity to begin with in the ambulatory control conditions. In contrast to hindlimb muscles, LPL activity in the heart and diaphragm remained unchanged during HU (Fig. 2). This could be interpreted simply by the fact that the heart and diaphragm must continue working even during reduced weight-bearing activity, and thus local signals for TG-derived fatty acid utilization remained intact.
The large decrease in LPL in hindlimb muscles during inactivity was independent of a change in LPL mRNA concentration. LPL mRNA concentration remained unchanged in the soleus or RF muscles after both acute and prolonged intermittent (10 h day−1 for 11 days) inactivity (Table 3). The present data confirm and extend the recent finding with other methodologies that 12 h of inactivity in the soleus did not change LPL mRNA (Bey et al. 2003). We do not know whether more than 11 days would eventually decrease LPL mRNA significantly. However, even if this happened, the fact that the mRNA change would follow the more rapid decrease in LPL activity observed after just 1 day implies that the pretranslational mechanisms may not be causative, but merely associative or additive. Even though ageing is the most chronic condition causing physical inactivity, ageing decreases LPL activity and protein in the oxidative soleus muscle without any change in LPL mRNA concentration (Bey et al. 2001). As a caveat, we reported that unloading muscle (24 h day−1) by casting (Hamilton et al. 1998) or hindlimb suspension (present study) decreased LPL mRNA concentration. However, in the rat (but not human) profound muscle wasting takes place in 1 week of continuous unloading (Howard et al. 1989; Riley et al. 1990) that involves a large loss of both the muscle mass and total RNA (-56 %). Such effects were not occurring with prolonged intermittent inactivity. Also, because LPL activity and protein decreased at 12 h and the LPL mRNA was slower in decreasing at 7 days of continuous HU, it is not reasonable to suspect that the cause of the decrease in muscle LPL activity during continuous inactivity was a decrease in mRNA concentration. Instead we suggest that this decrease in LPL mRNA was a secondary consequence of further muscle adaptations induced by continuous inactivity, perhaps related to muscle wasting and remodelling.
The potential for modulating LPL mRNA concentration is apparently dependent on fibre type recruitment and/or exercise intensity. Relatively strenuous exercise can increase LPL mRNA concentration (Seip et al. 1997; Hamilton et al. 1998) locally in glycolytic fibre types (Hamilton et al. 1998). Lower intensity treadmill or voluntary walking is apparently sub-threshold for this exercise response, as in the present study. Simsolo et al. (1993) concluded that de-training of active people leads to a profound loss of skeletal muscle LPL activity, largely because of post-translational processes. Thus, the stable LPL mRNA concentration and ActD results during treadmill walking suggest that LPL transcription is not essential for up-regulating LPL activity at the low end of the dose-response curve. It remains to be seen whether LPL mRNA induction during intense contractions is essential for raising LPL activity.
The timing of the LPL protein mass changes also provides clues about the underlying process. Note that at the early time of 6 h, HR-LPL protein had decreased by the same percentage as HR-LPL activity, but the total LPL protein (primarily intracellular) had still not decreased significantly (Table 4). This may mean that there is a process causing a loss of LPL protein at the endothelium or other heparin binding sites before the larger precursor pool of LPL inside cells is drained.
Researchers interested in discovering treatments for lipid disorders have aggressively sought to discover the physiological and molecular factors that might be necessary to optimally maintain LPL activity, especially tissue-specific LPL patterns. Thus, it is impressive to observe that nothing else has a more deleterious effect on muscle HR-LPL than inactivity and to note the powerful counteractive influence of moderate amounts of contractile activity. The findings with ActD (Table 5) suggest a nuclear event is involved in the signalling for the loss of LPL in muscles. It has been known for a long time that ActD can raise LPL activity in adipose tissue (Schotz & Garfinkel, 1965; Wing & Robinson, 1968; Bergo et al. 2002). The lack of effect of ActD on active muscles and, instead, a preventative effect in the inactive muscle, provides the interesting evidence that the LPL inhibitor gene(s) is only expressed during inactivity or that physical inactivity ‘sensitizes’ muscle to it. In contrast, the same transcriptional inhibitor did not have a significant effect either on the maintenance of high skeletal muscle LPL activity in the ambulatory control animals (Table 5) or on the large rise in the LPL activity induced by treadmill walking (data in text of Results).
LPL has a central role in lipid metabolism and has been studied in literally hundreds of papers, including potential effects on the local uptake of plasma TG into the underlying muscle, local alterations in muscle glucose and fatty acid metabolism, alterations in HDL cholesterol, obesity and downstream factors related to atherosclerosis in animals that develop CHD. There is still much to be learned about the biochemical effects of changes in HR-LPL activity and protein during inactivity, but the most easily interpreted result with the HU model is the local decrease in [3H]TG uptake (Fig. 3). Since LPL activity and TG-derived fatty acid uptake in muscle are apparently related to energy demand, it follows that inactive muscle would have low lipid uptake in order to minimize unnecessary lipid accumulation and potential lipotoxicity.
Fasting plasma TG concentration is often not different between alternating days with prolonged walking or resting when the TG values are already low, even if muscle LPL activity is changing manyfold (Lithell et al. 1981a). Also, it is important to remember that the muscle mass of a rat hindlimb is only ≈6 % of body weight. Others concluded that LPL reductions during bed-rest may be in part related to a ≈20 % reduction of HDL-C, and a doubling of the average fasting VLDL TG concentration (Yanagibori et al. 1997, 1998). Raising energy demand with even moderate exercise can produce marked improvements in hypertriglyceridemic people, and produce consistent enhancement of normal subjects when challenged by a fat load (Frandin et al. 1991; Aldred et al. 1994; Herd et al. 2001).
A consensus panel concluded that altered TG metabolism exemplifies the strongest category of evidence for disease related to inactivity (Kesaniemi et al. 2001; Bouchard, 2001), in part because of “the strong and consistent effects of even one day of activity [on metabolism], well before traditional standards of ‘physical fitness’” (Thompson et al. 2001). It appears that muscle LPL regulation is one of the most sensitive metabolic responses to physical inactivity and low-intensity contractile activity. This may provide one piece of the puzzle in offering a plausible explanation for how inactivity is related to chronic diseases and why small amounts of activity provide marked protection against CHD (Kramsch, 1981). Because of the potential role of fatty acid-derived TG in signalling, it is possible that these changes in LPL and TG uptake are part of more widespread changes in muscle metabolism, as indicated recently by a change in gene expression for over 100 genes after several hours of inactivity (Bey et al. 2003).
Acknowledgments
We thank A. Holloway, D. G. Hamilton and Dr T. Zderic for technical support and discussions. This study was supported by NIH (HL57367) and NSBRI (00119611) to M. T. Hamilton and LSME postdoctoral fellowship to L. Bey.
REFERENCES
- Aldred HE, Perry IC, Hardman AE. The effect of a single bout of brisk walking on postprandial lipemia in normolipidemic young adults. Metabolism. 1994;43:836–841. doi: 10.1016/0026-0495(94)90263-1. [DOI] [PubMed] [Google Scholar]
- Alford EK, Roy RR, Hodgson JA, Ergerton VR. Electromyography of rat soleus, medial gastrocnemius, and tibialis anterior during hindlimb suspension. Exp Neur. 1987;96:635–649. doi: 10.1016/0014-4886(87)90225-1. [DOI] [PubMed] [Google Scholar]
- Augustus AS, Kako Y, Yagyu H, Goldberg IJ. Routes of FA delivery to cardiac muscle: modulation of lipoprotein lipolysis alters uptake of TG-derived FA. Am J Physiol. 2003;284:E331–339. doi: 10.1152/ajpendo.00298.2002. [DOI] [PubMed] [Google Scholar]
- Bergo M, Wu G, Ruge T, Olivecrona T. Down-regulation of adipose tissue lipoprotein lipase during fasting requires that a gene, separate from the lipase gene, is switched on. J Biol Chem. 2002;277:11927–11932. doi: 10.1074/jbc.M200325200. [DOI] [PubMed] [Google Scholar]
- Bey L, Akunuri N, Hoffman PE, Zhao P, Hamilton DG, Hamilton MT. Patterns in global gene expression in rat skeletal muscle during unloading and low-intensity ambulatory activity. Physiol Genomics. 2003;13:157–167. doi: 10.1152/physiolgenomics.00001.2002. [DOI] [PubMed] [Google Scholar]
- Bey L, Areiqat E, Sano A, Hamilton MT. Reduced lipoprotein lipase activity in postural skeletal muscle during aging. J Appl Physiol. 2001;91:687–692. doi: 10.1152/jappl.2001.91.2.687. [DOI] [PubMed] [Google Scholar]
- Blair SN, Brodney S. Effects of physical inactivity and obesity on morbidity and mortality: current evidence and research issues. Med Sci Sports Exerc. 1999;31:S646–662. doi: 10.1097/00005768-199911001-00025. [DOI] [PubMed] [Google Scholar]
- Bouchard C. Physical inactivity and health: introduction to the dose-response symposium. Med Sci Sports Exerc. 2001;33:S347–350. doi: 10.1097/00005768-200106001-00002. [DOI] [PubMed] [Google Scholar]
- Colleran PN, Wilkerson MK, Bloomfield SA, Suva LJ, Turner RT, Delp MD. Alterations in skeletal perfusion with simulated microgravity: a possible mechanism for bone remodeling. J Appl Physiol. 2000;89:1046–1054. doi: 10.1152/jappl.2000.89.3.1046. [DOI] [PubMed] [Google Scholar]
- Frandin K, Grimby G, Mellstrom D, Svanborg A. Walking habits and health-related factors in a 70-year-old population. Gerontology. 1991;37:281–288. doi: 10.1159/000213272. [DOI] [PubMed] [Google Scholar]
- Goldberg IJ, Le NA, Ginsberg HN, Krauss RM, Lindgren FT. Lipoprotein metabolism during acute inhibition of lipoprotein lipase in the cynomolgus monkey. J Clin Invest. 1998;81:561–568. doi: 10.1172/JCI113354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg IJ, Merkel M. Lipoprotein lipase: physiology, biochemistry, and molecular biology. Front Biosci. 2001;6:D388–405. doi: 10.2741/goldberg. [DOI] [PubMed] [Google Scholar]
- Hamilton MT, Areiqat E, Hamilton DG, Bey L. Plasma triglyceride metabolism in humans and rats during aging and physical inactivity. Int J Sport Nutr Exerc Metab. 2001;11:S97–104. doi: 10.1123/ijsnem.11.s1.s97. [DOI] [PubMed] [Google Scholar]
- Hamilton MT, Booth FW. Skeletal muscle adaptation to exercise: a century of progress. J Appl Physiol. 2000;88:327–331. doi: 10.1152/jappl.2000.88.1.327. [DOI] [PubMed] [Google Scholar]
- Hamilton MT, Etienne J, McClure WC, Pavey BS, Holloway AK. Role of local contractile activity and muscle fiber type on LPL regulation during exercise. Am J Physiol. 1998;275:E1016–1022. doi: 10.1152/ajpendo.1998.275.6.E1016. [DOI] [PubMed] [Google Scholar]
- Haskell WL. Health consequences of physical activity: understanding and challenges regarding dose-response. Med Sci Sports Exerc. 1994;26:649–660. doi: 10.1249/00005768-199406000-00001. [DOI] [PubMed] [Google Scholar]
- Henderson HE, Kastelein JJ, Zwinderman HH, Gagne E, Jukema JW, Reymer PW, Groenemeyer BE, Lie KI, Bruschke AV, Hayden MR, Jansen H. Lipoprotein lipase activity is decreased in a large cohort of patients with coronary artery disease and is associated with changes in lipids and lipoproteins. J Lipid Res. 1999;40:735–743. [PubMed] [Google Scholar]
- Hennig R, Lomo T. Firing patterns of motor units in normal rats. Nature. 1985;314:164–166. doi: 10.1038/314164a0. [DOI] [PubMed] [Google Scholar]
- Herd SL, Kiens B, Boobis LH, Hardman AE. Moderate exercise, postprandial lipemia, and skeletal muscle lipoprotein lipase activity. Metabolism. 2001;50:756–762. doi: 10.1053/meta.2001.24199. [DOI] [PubMed] [Google Scholar]
- Howard G, Steffen JM, Geoghegan TE. Transcriptional regulation of decreased protein synthesis during skeletal muscle unloading. J Appl Physiol. 1989;66:1093–1098. doi: 10.1152/jappl.1989.66.3.1093. [DOI] [PubMed] [Google Scholar]
- Kesaniemi YK, Danforth E, Jr, Jensen MD, Kopelman PG, Lefebvre P, Reeder BA. Dose-response issues concerning physical activity and health: an evidence-based symposium. Med Sci Sports Exerc. 2001;33:S351–358. doi: 10.1097/00005768-200106001-00003. [DOI] [PubMed] [Google Scholar]
- Kramsch DM, Aspen AJ, Abramowitz BM, Kreimendahl T, Hood WB. Reduction of coronary atherosclerosis by moderate conditioning exercise in monkeys on an atherogenic diet. N Eng J Med. 1981;305:52–57. doi: 10.1056/NEJM198112173052501. [DOI] [PubMed] [Google Scholar]
- Ladu MJ, Kapsas H, Palmer WK. Regulation of lipoprotein lipase in muscle and adipose tissue during exercise. J Appl Physiol. 1991;71:404–409. doi: 10.1152/jappl.1991.71.2.404. [DOI] [PubMed] [Google Scholar]
- Levak-Frank S, Weinstock PH, Hayek T, Verdery R, Hofmann W, Ramakrishnan R, Sattler W, Breslow JL, Zechner R. Induced mutant mice expressing lipoprotein lipase exclusively in muscle have subnormal triglycerides yet reduced high density lipoprotein cholesterol levels in plasma. J Biol Chem. 1997;272:17182–17190. doi: 10.1074/jbc.272.27.17182. [DOI] [PubMed] [Google Scholar]
- Linder C, Chernick SS, Fleck TR, Scow RO. Lipoprotein lipase and uptake of chylomicrons triglyceride by skeletal muscle of rats. Am J Physiol. 1976;231:860–864. doi: 10.1152/ajplegacy.1976.231.3.860. [DOI] [PubMed] [Google Scholar]
- Lithell H, Cedermark M, Froberg J, Tesch P, Karlsson J. Increase of lipoprotein lipase activity in skeletal muscle during heavy exercise. Relation to epinephrine excretion. Metabolism. 1981a;30:1130–1134. doi: 10.1016/0026-0495(81)90059-7. [DOI] [PubMed] [Google Scholar]
- Lithell H, Lindgarde F, Nygaard E, Saltin B. Capillary supply and lipoprotein lipase activity in skeletal muscle in man. Acta Physiol Scand. 1981b;111:383–384. doi: 10.1111/j.1748-1716.1981.tb06753.x. [DOI] [PubMed] [Google Scholar]
- McDonald KS, Delp MD, Fitts RH. Effect of hindlimb unweighting on tissue blood flow in the rat. J Appl Physiol. 1992;72:2210–2218. doi: 10.1152/jappl.1992.72.6.2210. [DOI] [PubMed] [Google Scholar]
- Mackie BG, Dudley GA, Kaciuba-Uscilko H, Terjung RL. Uptake of chylomicron triglycerides by contracting skeletal muscle in rats. J Appl Physiol. 1980;49:851–855. doi: 10.1152/jappl.1980.49.5.851. [DOI] [PubMed] [Google Scholar]
- Martins MC, Pileggi F, Maranhao RC. Clearance of a chylomicron-like emulsion from plasma is delayed in patients with coronary artery disease. Braz J Med Biol Res. 1995;28:427–431. [PubMed] [Google Scholar]
- Morris JN, Heady JA, Raffle PAB. Coronary heart disease and physical inactivity of work. Lancet. 1953;1053:1111. doi: 10.1016/s0140-6736(53)91495-0. [DOI] [PubMed] [Google Scholar]
- Olivecrona T, Hultin M, Bergo M, Olivecrona G. Lipoprotein lipase: regulation and role in lipoprotein metabolism. Proc Nutr Soc. 1997;56:723–729. doi: 10.1079/pns19970072. [DOI] [PubMed] [Google Scholar]
- Riley DA, Slocum GR, Bain JL, Sedlak FR, Sowa TE, Mellender JW. Rat hindlimb unloading: soleus histochemistry, ultrastructure, and electromyography. J Appl Physiol. 1990;69:58–66. doi: 10.1152/jappl.1990.69.1.58. [DOI] [PubMed] [Google Scholar]
- Seip RL, Mair K, Cole TG, Semenkovich CF. Induction of human skeletal muscle lipoprotein lipase gene expression by short-term exercise is transient. Am J Physiol. 1997;272:E255–261. doi: 10.1152/ajpendo.1997.272.2.E255. [DOI] [PubMed] [Google Scholar]
- Schlaepfer IR, Eckel RH. Plasma triglyceride reduction in mice after direct injections of muscle-specific lipoprotein lipase DNA. Diabetes. 1999;48:223–227. doi: 10.2337/diabetes.48.1.223. [DOI] [PubMed] [Google Scholar]
- Schotz MC, Garfinkel AS. The effect of puromycin and actinomycin on carbohydrate-induced lipase activity in rat adipose tissue. Biochim Biophys Acta. 1965;106:202–205. doi: 10.1016/0005-2760(65)90109-8. [DOI] [PubMed] [Google Scholar]
- Simsolo RB, Ong JM, Kern PA. The regulation of adipose tissue and muscle lipoprotein lipase in runners by detraining. J Clin Invest. 1993;92:2124–2130. doi: 10.1172/JCI116813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smol E, Zernicka E, Czarnowski D, Langfort J. Lipoprotein lipase activity in skeletal muscles of the rat: effects of denervation and tenotomy. J Appl Physiol. 2001;90:954–960. doi: 10.1152/jappl.2001.90.3.954. [DOI] [PubMed] [Google Scholar]
- Thompson PD, Crouse SF, Goodpaster B, Kelley D, Moyna N, Pescatello L. The acute versus the chronic response to exercise. Med Sci Sports Exerc. 2001;33:S438–445. doi: 10.1097/00005768-200106001-00012. [DOI] [PubMed] [Google Scholar]
- Wing DR, Robinson DS. Clearing-factor lipase in adipose tissue. Studies with puromycin and actinomycin. Biochem J. 1968;106:667–676. doi: 10.1042/bj1060667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittrup H, Tybjaerg-Hansen A, Nordestgaard BG. Lipoprotein lipase mutations, plasma lipids and lipoproteins, and risk of ischemic heart disease. A meta-analysis. Circulation. 1999;99:2901–2907. doi: 10.1161/01.cir.99.22.2901. [DOI] [PubMed] [Google Scholar]
- Yanagibori R, Kondo K, Suzuki Y, Kawakubo K, Iwamoto T, Itakura H, Gunji A. Effect of 20 days bed rest on the reverse cholesterol transport system in healthy young subjects. J Intern Med. 1998;243:307–312. doi: 10.1046/j.1365-2796.1998.00303.x. [DOI] [PubMed] [Google Scholar]
- Yanagibori R, Suzuki Y, Kawakubo K, Kondo K, Iwamoto T, Itakura H, Makita Y, Sekiguchi C, Gunji A, Kondou K. The effects of 20 days bed rest on serum lipids and lipoprotein concentrations in healthy young subjects. J Gravit Physiol. 1997;4:S82–90. [PubMed] [Google Scholar]


