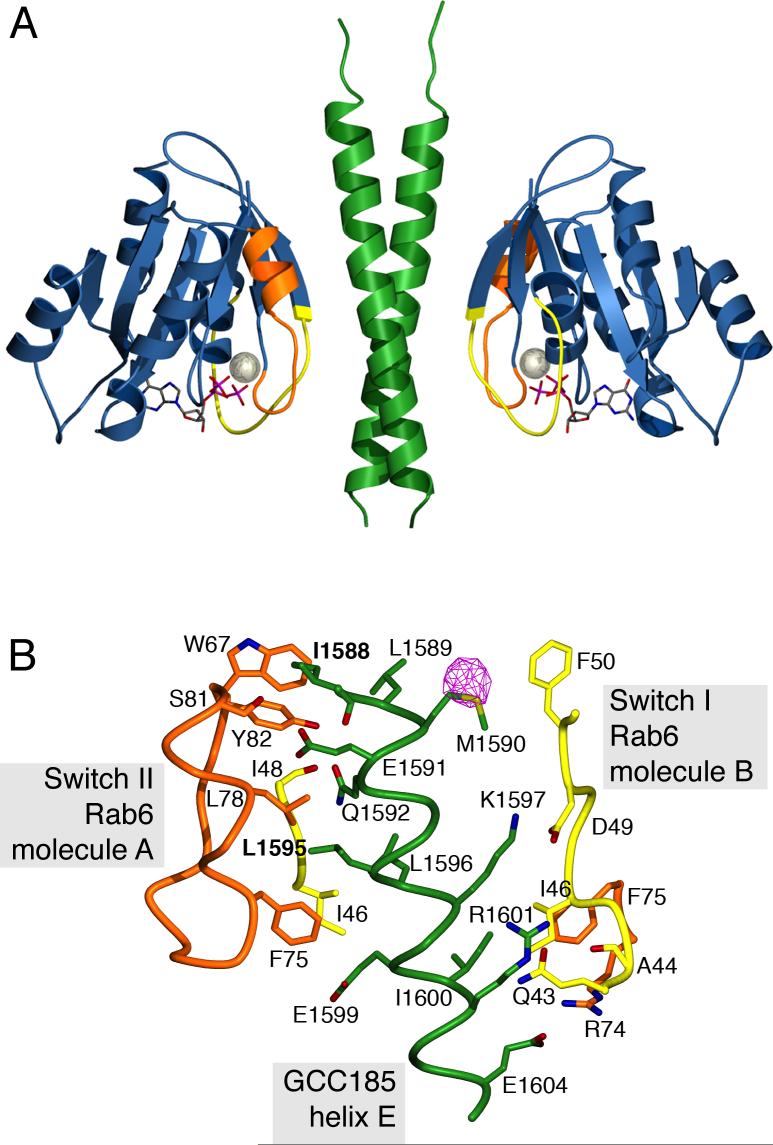
Figure 4.
Structure of the Rab6-GCC185 complex. A. Ribbon representation of the GCC185 Rab binding domain dimer (green) and Rab6 (blue) bound to GTP (stick model) and magnesium (sphere). Switch I and II regions of Rab6 (Chattopadhyay et al., 2000) are colored yellow and orange respectively. B. View of the Rab6-GCC185 binding interface. A single GCC185 helix (E) out of the two-fold symmetric coiled coil is shown for clarity. Each helix contacts switch regions from two opposed Rab6 molecules A and B. Rab6 switch I and II (including W67), are colored yellow and orange, respectively. Protein backbone (α-carbon trace) and side chains involved in polar and hydrophobic interactions are shown. Carbonyl oxygens are shown for A44, I48 and I1588 and C-Cα bonds have been added to simplify the figure. An anomalous difference Fourier density map of the selenomethionine substituted crystal (pink, contoured at 6σ) is shown for GCC185.