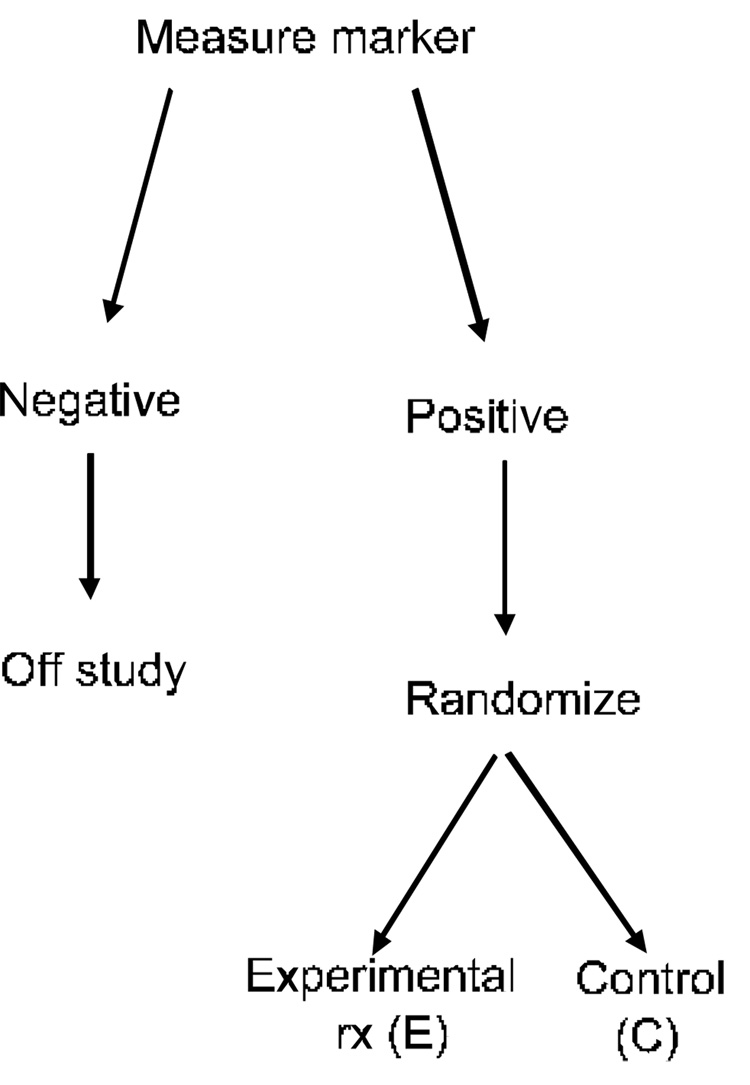
Figure 3.
Targeted clinical trial design for evaluating a new experimental therapy. A biomarker classifier is developed for identifying those patients most likely to respond to the new treatment (E). Only those patients are randomized to E versus the control treatment. The patients predicted less likely to respond (marker negative) are off study. The targeted design is most useful in cases where the biomarker classifier has a strong biological rationale for identifying responsive patients and where it may not be ethically advisable to expose marker negative patients to the new treatment.