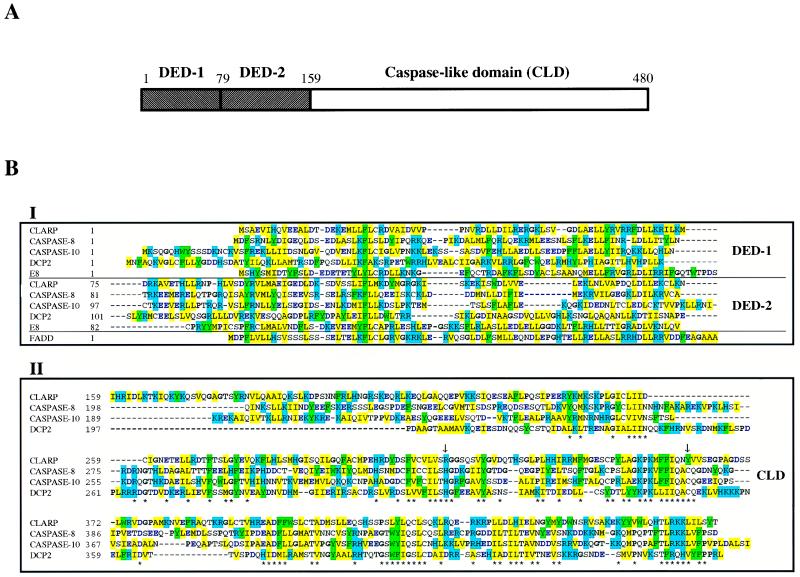
Figure 1.
Structure, sequence, and alignment of CLARP with related proteins. (A) Schematic structure of human CLARP. DEDs and caspase-like domain (CLD) are shown as hatched and empty boxes. (B) The amino acid sequence of CLARP is aligned with those of human caspase-8 (accession no. 1401352), human caspase-10 (1498324), DCP-2 (U20542), E8 protein of equine herpesvirus-2 (1360817), and DED of FADD (1082361). DEDs and CLD are shown in box I and box II, respectively. Homologous amino acid residues are highlighted with same colors; black bold for P and G (αβ breaker), blue bold for D and E (acidic residues), blue background for R, K, and H (positive charged residues), green background for W, F, and Y (aromatic residues), and yellow background for I, L, V, A, and M (hydrophobic residues). The two essential catalytic residues are indicated by arrows. Conserved residues among more than three proteins are indicated by stars for box II. The entire nucleotide sequence of CLARP cDNA is available as accession no. AF005774 in GenBank.