Fig. 1.
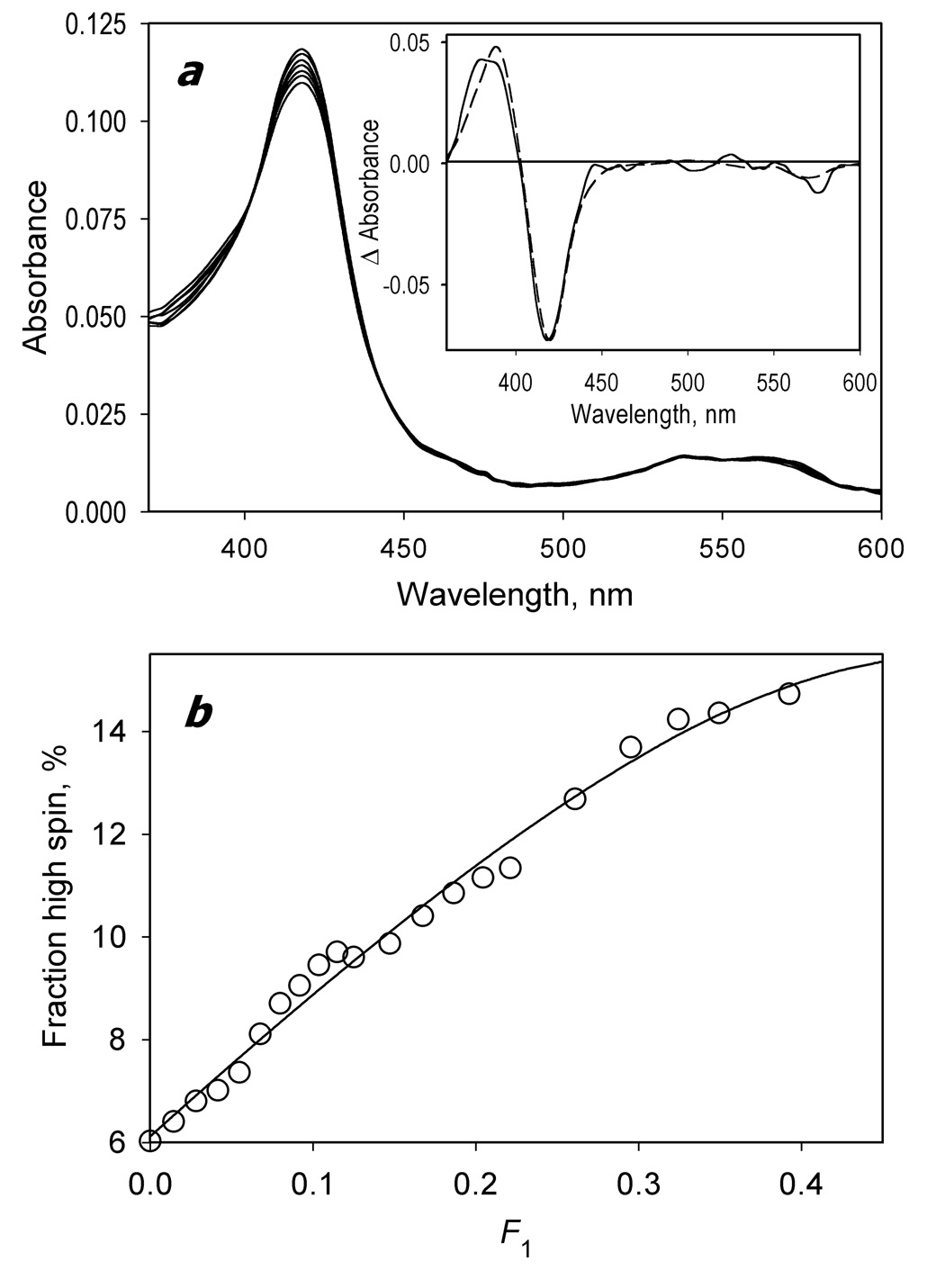
Formation of the CYP3A4 complex with BMR studied by a counter-flow continuous variation technique. At the beginning of the experiment the first and the second compartments of a 2 × 5 mm tandem cell contained equal volumes of 2.8 µM solutions of BMR and CYP3A4, respectively. The interactions were monitored by the changes in the concentration of P450 low-spin and high-spin states. Mixing of the interacting proteins was achieved by reciprocal gradual displacement of small aliquots of the solutions between compartments, so that the molar fraction of CYP3A4 in the first compartment (F1) changed from 0 to 0.5. The experiment was performed at 25 °C in 0.1 mM Na-HEPES buffer (pH 7.4) containing 1 mM EDTA and 1 mM DTT. A series of absorbance spectra recorded at F1 values of 0, 0.08, 0.22, 0.32, 0.42, 0.48, and 0.49 are shown in panel a. The inset shows the spectrum of the first principal component of the observed changes found by PCA (solid line). The dashed line in the inset represents the standard spectrum of the CYP3A4 high-to-low-spin shift. Panel b shows the dependence of the high-spin fraction of CYP3A4 on F1 obtained from the analysis of the spectra. The solid line represents the results of the fitting of the titration curve to the equation for the equilibrium of bimolecular association suited to the case of the counter-flow titration (eq. (3) in [19]).