Abstract
Fertility and fecundity decline with advancing age in female mammals, but reproductive aging was decelerated in Siberian hamsters (Phodopus sungorus) raised in a short day (SD) photoperiod. Litter success was significantly improved in older hamsters when reared in SD and the number of primordial follicles was twice that of females held in long days (LD). Because anti-Müllerian hormone (AMH) appears to inhibit the recruitment of primordial follicles in mice, we sought to determine if the expression patterns of AMH differ in the ovaries and serum of hamsters raised in SD versus LD. Ovaries of SD female hamsters are characterized by a paucity of follicular development beyond the secondary stage and are endowed with an abundance of large eosinophilic cells, which may derive from granulosa cells of oocyte-depleted follicles. In ovaries from 10 week-old SD hamsters, we found the so-called “hypertrophied granulosa cells” were immunoreactive for AMH, as were granulosa cells within healthy appearing primary and secondary follicles. Conversely, ovaries from age-matched LD animals lack the highly eosinophilic cells present in SD ovaries. Therefore, AMH staining in LD was limited to primary and secondary follicles, which are comparable in number to those found in SD ovaries. The substantially greater AMH expression in SD ovaries probably reflects the abundance of hypertrophied granulosa cells in SD ovaries and their relative absence in LD ovaries. The modulation of ovarian AMH by day length is a strong mechanistic candidate for the preservation of primordial follicles in female hamsters raised in a SD photoperiod.
Keywords: anti-Mullerian hormone, Ovary, Photoperiod, hamster
Introduction
The mammalian ovary is endowed with a limited number of germ cells, and at any given point in a female’s lifetime the number of primordial follicles represents the resting pool of oocytes from which she can draw upon for future reproductive efforts (Hirshfeld 1994). Continuous activation, or recruitment, of primordial follicles over time results in their numerical decline with age, and in women, menopause ensues when near or complete exhaustion of ovarian follicles occurs (Faddy & Gosden 1994). Several hormones and factors have been shown to modulate primordial follicle activation and most of these have been found to be stimulatory (reviewed in Fortune 2003). Conversely, there is convincing evidence that anti-Müllerian hormone (AMH, also known as Müllerian inhibiting substance or MIS) suppresses primordial follicle activation via autocrine and paracrine effects (Durlinger et al. 1999, 2002a). Until relatively recently, AMH was best known for its expression in Sertoli cells of developing testes and the regression of the Müllerian ducts in males during sexual differentiation (Jost 1947). However, differentiated ovaries also produce AMH and the granulosa cells of growing preantral follicles appear to be the principal source (Ueno et al. 1989, Hirobe et al. 1992, Baarends et al. 1995, Durlinger et al., 2002a, 2002b).
In female Siberian hamsters (Phodopus sungorus), short day (SD) conditions profoundly inhibit reproductive physiology and development (Ebling 1994, Adam et al. 2000), and the number of ovarian primordial follicles appears to be influenced by the photoperiod in which females are reared (Place et al. 2004, Timonin et al. 2006). Because AMH suppresses primordial follicle activation, we investigated the effects of day length on ovarian AMH expression in Siberian hamsters. Ovaries from females held in SD have an unusual feature – granulosa cells from preantral follicles appear to luteinize following atresia of the oocyte (van den Hurk et al. 2002, Place et al. 2004, Timonin et al. 2006) and the histology of these luteinized granulosa cells is consistent with continued steroidogenic activity (van den Hurk et al. 2002, Place et al. 2004). To determine if these granulosa cells continue to express AMH after oocyte atresia, we performed immunohistochemistry on ovaries from hamsters raised in either SD or long days (LD). Luteinized granulosa cells of atretic follicles within SD ovaries were immunoreactive for AMH, and because these cells account for a substantial portion of the SD ovary’s volume, we predicted AMH protein expression would be greater in whole ovaries from SD as compared to LD hamsters.
Circulating levels of AMH decline with age, which may reflect the age-associated depletion of ovarian follicles (Kevenaar et al. 2006). Interestingly, serum AMH concentrations correlate with the number of ovarian primordial follicles in mice (Kevenaar et al. 2006) and women (Bath et al. 2003). Measurement of serum AMH concentration may be a useful marker when assessing a woman’s probability of success when considering assisted reproductive technologies that require gonadotropin stimulation and collection of preovulatory oocytes (e.g., in vitro fertilization) (van Rooij et al. 2005). The number of primordial follicles within ovaries from Siberian hamsters raised in SD was approximately twice the number seen in age-matched LD ovaries (Place et al. 2004, Timonin et al. 2006), thus we measured serum AMH concentrations to test our prediction that circulating AMH would be higher in SD than LD hamsters.
Methods and Materials
Experimental Animals
Siberian hamsters from our colony (14 hours of light per day, 14L) were transferred to LD (16L) or SD (10L) as breeding pairs to generate females for this study. The time of lights-off was synchronized for all animals to 1700 Eastern Standard Time (EST). Animals were originally derived from wild-bred stock obtained from Dr. K. Wynne-Edwards, Queen’s University. Experimental females were weaned on postnatal day 18, placed in polypropylene cages (2 to 4 siblings/cage), and maintained in the photoperiod in which they were born. Food (Teklad 8626, Madison, WI) and water were available ad libitum. Ambient temperature and relative humidity were held constant between 21°C ± 5 and 50 ± 10%, respectively.
Blood and Tissue Collection
Fourteen LD and 14 SD females were given an intraperitoneal overdose of sodium pentobarbital, weighed, and then exsanguinated by retro-orbital bleed at 10 weeks of age. All animals were euthanized during the middle of the light cycle, between 1200 and 1400 EST. Blood was clotted on ice for at least 1 hr and centrifuged at 3,600 rpm for 20 min in 4°C. Drawn off serum was frozen and maintained at −80°C until assayed for AMH.
Both ovaries were removed from each animal, dissected free of surrounding fat, and weighed on an analytical balance. One of the ovaries, selected pseudo-randomly from the right or left side, was placed in protein extraction buffer (10 mM Tris, 0.5M NaCl, 1mM MgCl2, 0.1% Triton X100, 1 tablet/10ml Complete Mini Protease Inhibitor [Roche, Indianapolis, IN]) in preparation for Western blots. The remaining ovary from six animals in each group was immersed in 10% buffered formalin for histology and follicle counts, and half of those ovaries, three from each group, were used for AMH immunohistochemistry. Formalin fixation continued overnight at room temperature, followed by serial dehydration into 70% ethanol. Ovaries were embedded in paraffin and serially sectioned at 6 μm. Every tenth section was stained with hematoxylin and eosin and viewed under 400X magnification to count the types of ovarian follicles that express AMH with the greatest intensity, i.e., primary and secondary follicles. Because AMH is principally expressed in granulosa cells, we further subdivided secondary follicles into categories with less than 4 layers of granulosa cells (types 4 and 5a), or 4 or more layers of granulosa cells (type 5b) surrounding the oocyte (Pedersen & Peters 1968). Primary follicles were defined as an oocyte surrounded by a single layer of cuboidal granulosa cells.
Immunohistochemistry for AMH
Six mid-ovary sections from each of three animals in each group were immunostained for AMH. Sections from LD and SD ovaries were alternately placed on each slide to control for potential staining variability between slides. Adjacent sections were mounted on separate slides for negative controls. After dewaxing and rehydration in a series of ethanols, endogenous peroxides were quenched in hydrogen peroxide (0.3% in methanol) for 30 min. Sections were then incubated in 10% normal rabbit serum diluted in dilution buffer (0.5M Sodium chloride, 0.01M Phosphate Buffer, 3% BSA, 0.3% Triton-X 100) for 20 min at room temperature to block nonspecific binding sites. Polyclonal goat anti-MIS antibody (sc-6886, Santa Cruz Biotechnology, Santa Cruz, CA) was diluted 1:1000 in dilution buffer and incubated with sections for 16 hours at 4°C. As a negative control, primary antibody was incubated 1:1 overnight with MIS (C-20) Blocking Peptide (sc-6886P, Santa Cruz Biotechnology, Santa Cruz, CA) on a rocker at 4°C before its application to sections as described above. Additional negative controls excluded the primary or secondary antibody. Sections were incubated with the secondary antibody, biotinylated rabbit anti-goat IgG (Santa Cruz Biotechnology; 1:200 in dilution buffer), for 30 min. Immunoreactivities were visualized by incubating sections with Vectastain Elite ABC Solution (Vector, Burlingame, CA) for 30 min and developed with NovaRed Peroxidase Substrate Solution (Vector, Burlingame, CA) following the manufacturer’s instructions. Sections were counterstained with hematoxylin.
Western blot for AMH
Freshly collected ovaries were homogenized in protein extraction buffer, and protein concentrations were determined with the DC Protein Assay Kit (Bio-Rad, Hercules, CA). For each sample, approximately 10 μg of protein were resolved on a 10% SDS gel under reducing conditions, followed by transfer onto a nitrocellulose membrane (Bio-Rad, Hercules, CA). The membrane was incubated in SuperBlock Blocking Buffer in TBS (Pierce, Rockford, IL) for 60 min at room temperature to block nonspecific binding sites. Polyclonal goat anti-MIS antibody (sc-6886, Santa Cruz Biotechnology, Santa Cruz, CA) was diluted 1:1000 in SuperBlock Blocking Buffer and incubated overnight at 4°C on a rocker. Specific binding was detected using horse peroxidase anti-goat secondary antibody (PI-9500, Vector, Burlingame, CA) at a dilution of 1:50,000. Labeled proteins were visualized by SuperSignal West Pico Chemiluminescents Substrate (Pierce, Rockford, IL) and viewed by autoradiography. Using the direct reprobing method outlined in Liao et al. (2000), the relative intensity of AMH was determined using β-actin as the loading control. Briefly, the blot was incubated for 1 hr at room temperature with monoclonal mouse anti-β-actin (Clone AC-150; Sigma, St. Louis, MO) diluted 1:5000 in SuperBlock Blocking Buffer, then probed for 1 hr at room temperature with goat peroxidase anti-mouse secondary antibody (31430, Pierce, Rockford, IL) at a dilution of 1:100,000. Labeled proteins were visualized as stated above.
Western blot films were scanned into a MacIntosh computer and the optical density of AMH and β-actin were analyzed with imaging processing software (ImageJ 1.34s, NIH, Bethesda, MD). AMH immunoreactivity was expressed relative to β-actin for each ovary.
ELISA for AMH
AMH was measured in duplicate serum samples using an enzyme-linked immunosorbent assay (ELISA) produced by Diagnostic Systems Laboratories (Webster, TX). This ELISA was validated for the measurement of AMH in Siberian hamsters by serially diluting a hamster serum sample in the kit’s sample diluent and demonstrating parallelism with the standard curve (range 0.05 to 15 ng/ml). The undiluted sample had a starting concentration of 4.26 ng/ml. All samples were run in a single assay and the intra-assay coefficient of variation was 22.0%. The minimum detection limit of the assay as reported by the manufacturer was 0.006 ng/ml.
Statistical analysis
Results were analyzed with a commercial statistical program (JMP version 5.1.2, SAS Institute, Cary, NC). LD v. SD comparisons of body and paired ovarian mass were made with t-tests, as were comparisons of ovarian and serum AMH. Uterine mass data were not normally distributed and the LD and SD variances were statistically different, thus the Median test was used for the uterine mass comparison. Because our preliminary data included animals from the same litter, we averaged all data from females within the same litter and treated the mean as a single data point. Thus, sample sizes reflect the number of litters represented, and litters in both groups contained from one to five females. We repeated all analyses using values from all individuals, and the statistical findings were identical to litter-based analyses. Pearson product-moment was used to determine if serum AMH concentration (by ELISA) correlated with ovarian AMH expression (by Western blot). Differences at p < 0.05 were considered to be significant.
Results
Body, uterine, and paired ovarian mass
When animals were killed at 10 weeks of age, females held in LD weighed significantly more than SD females. Uterine mass and paired ovarian mass were also greater in LD than in SD females (Table 1). Based on body, uterine, and paired ovarian mass, none of the SD females appeared to be photononresponders.
Table 1.
Body, uterine, and paired ovarian mass in Siberian hamsters reared in long or short days.
| Long Day (n = 7) | Short Day (n = 7) | p – value | |
|---|---|---|---|
| Body mass (g) | 29.8 ± 1.2 | 20.8 ± 1.2 | 0.002a |
| Uterine mass (mg) | 126.1 ± 26.2 | 15.0 ± 4.1 | < 0.001b |
| Paired ovarian mass (mg) | 9.7 ± 0.4 | 6.4 ± 0.7 | 0.003a |
Students t-test
Median test
Ovarian Histology and Immunohistochemistry
Ovarian histology was noticeably different in females raised in SD as compared to LD. Antral follicles were commonplace and corpora lutea (CL) were often present in LD (Fig. 1A), but not SD ovaries (Fig. 1B) (Table 2). Follicle development rarely advanced beyond the secondary stage in SD ovaries, and the SD ovary was also characterized by an abundance of hypertrophied eosinphilic cells, which surround atretic oocytes (Fig 1B).
Figure 1.
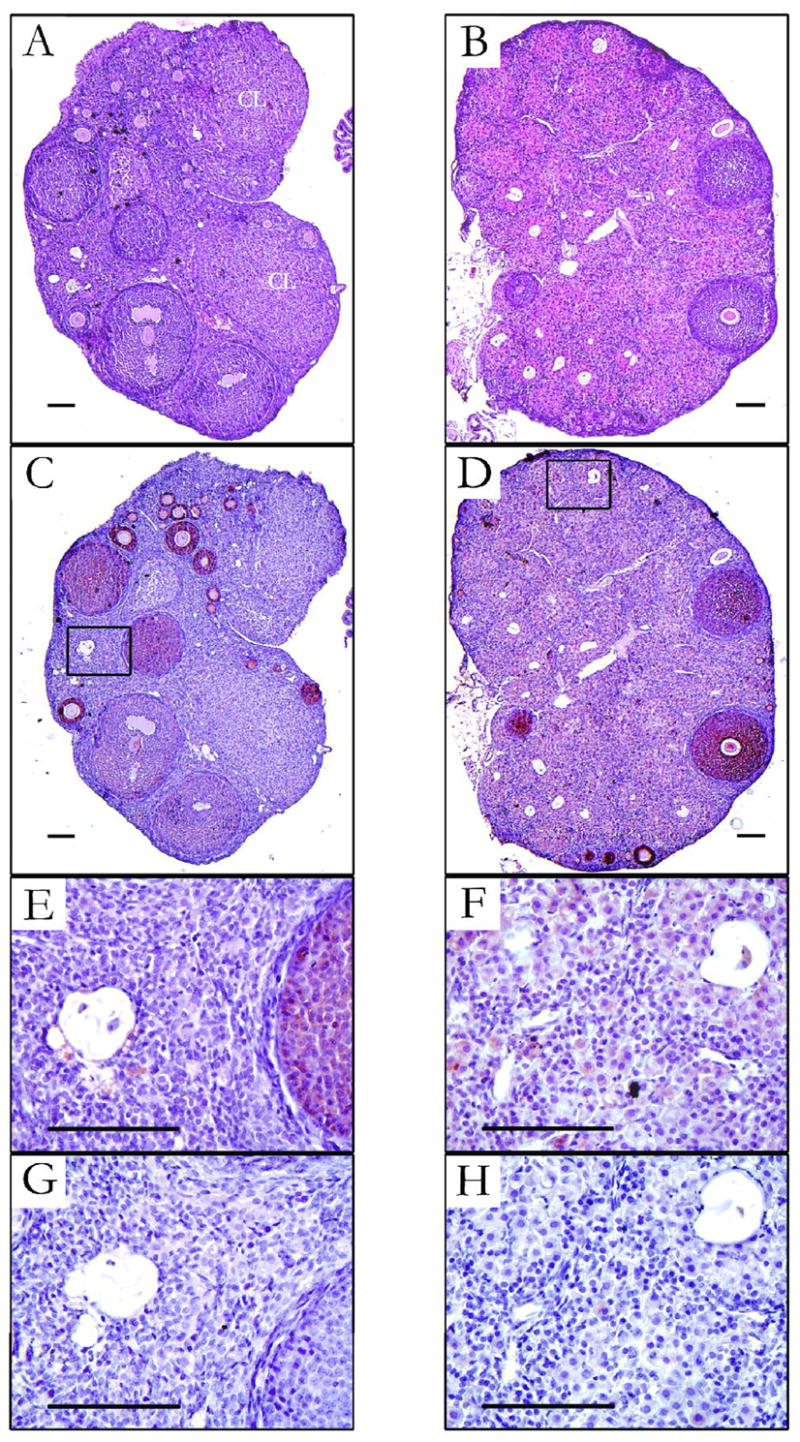
Photomicrographs of ovaries from Siberian hamsters held in either long days (LD; left panels) or short days (SD; right panels) from conception to 10 wk of age. (A and B) Hematoxylin and eosin staining. Corpora lutea (CL) were seen only in LD ovaries. (C and D) Immunhistochemistry for anti-Müllerian hormone (AMH) showing the most intense staining in granulosa cells in primary and secondary follicles. (E and F) Higher magnification of boxed regions in C and D, showing oocyte remnants and cells that surround them. Granulosa cells surrounding atretic oocytes in SD (F) have hypertrophied (luteinized) and stain positive for AMH, in contrast to comparable cells in LD ovaries (E). (G and H) Sections adjacent to those shown in E and F for which the primary antibody against AMH was pre-incubated with the blocking peptide – refer to methods for details. Bars = 100 μm.
Table 2.
Ovarian follicle counts in Siberian hamsters reared in long or short days.
| Day length | Primary | Secondary | Antral | Corpora luteac | |
|---|---|---|---|---|---|
| Types 4 & 5a | Type 5b | ||||
| Long (16L) | 25.2±3.6 | 17.3±2.1* | 6.8±0.9 | 2.2±0.5a | 3/6 |
| Short (10L) | 34.3±4.5 | 8.2±1.8 | 4.8±1.5 | 0.3±0.2b | 0/6 |
Five of six LD females had at least one antral follicle.
Two of six SD females had one antral follicle, the remaining four females had none.
Number of females in each group that had at least one corpus luteum.
Long day significantly greater than SD (p < 0.05).
Granulosa cells within primary and secondary follicles in both LD and SD ovaries showed the most intense staining for AMH following immunohistochemistry (Fig. 1C and D). AMH seemed to be less intense in the granulosa cells of antral follicles and absent in CL (Fig. 1C). AMH staining of moderate intensity was seen in the hypertrophied, or “luteinized”, granulosa cells that surround atretic oocytes in SD ovaries (Fig. 1F), whereas the cells surrounding atretic oocytes in LD ovaries were neither luteinized nor positive for AMH (Fig 1E). Pre-incubation of the antisera for AMH with blocking peptide before immunohistochemistry (Fig. 1G and H) confirmed the specificity of the AMH staining in the luteinized granulosa cells in SD ovaries. Non-specific AMH staining was not seen in negative controls, i.e., when the primary or secondary antibody was omitted, or when normal serum was used in substitution for the primary antibody (not shown). AMH immunohistochemistry of neonatal Siberian hamster testes revealed staining limited to Sertoli cells, as expected (not shown).
Because the most intense AMH staining was seen in primary and secondary follicles, we counted the numbers of these types of follicles and found greater numbers of small secondary follicles (types 4 and 5a) in LD as compared to SD ovaries, but no significant difference in the numbers of primary or large secondary (type 5b) follicles (Table 2).
Western blots
Western blot for AMH protein showed a single band in both LD and SD ovaries at the expected size (~ 70 kDa) (Fig. 2A). Whereas AMH expression was minimal to moderate in LD ovaries, AMH in SD ovaries was consistently moderate to high. Mean AMH protein level relative to β-actin expression was more than 3-fold higher in SD than in LD ovaries (Fig 2B). Because CLs can occupy a substantial volume of LD ovaries and do not express AMH (see Fig. 1A and C), we tried to determine if the presence of CLs in the ovary, as determined by histology, predicted lower AMH expression by Western blot in the contralateral ovary. With small sample sizes (3 LD ovaries with CLs and 3 without), which limited statistical power, we could detect no clear association between presence or absence of CLs and AMH expression of the ovary as a whole.
Figure 2.
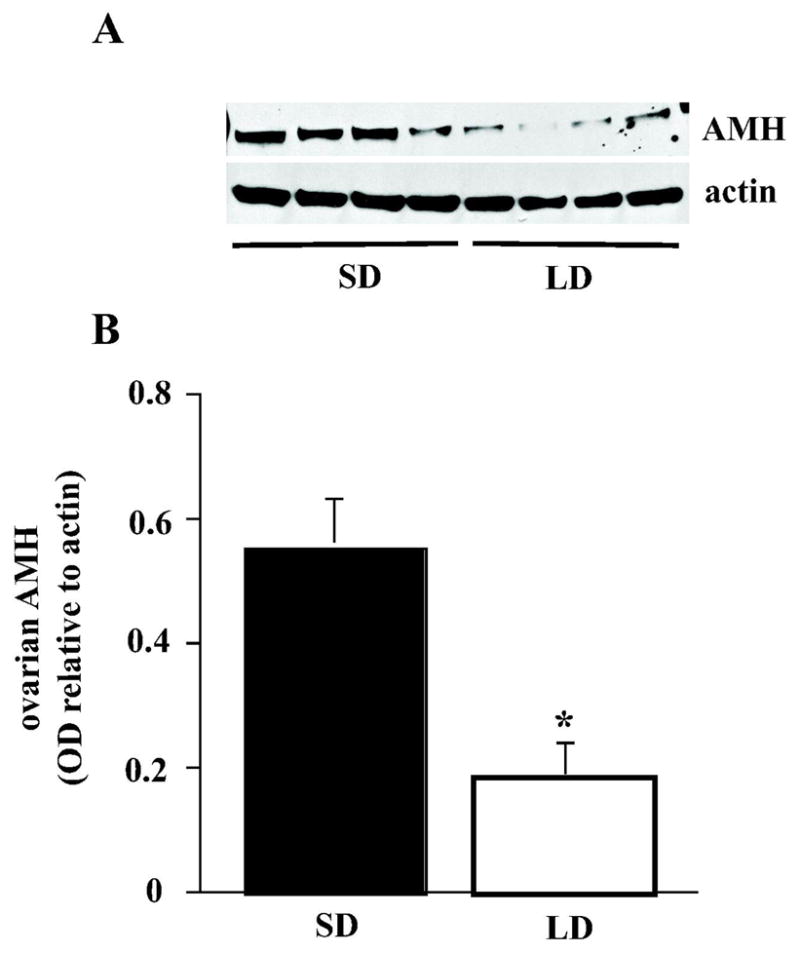
(A) Representative Western blots of ovarian homogenates from Siberian hamsters held in either long days (LD) or short days (SD) from conception to 10 wk of age. Anti-Müllerian hormone (AMH) and β-actin were blotted for each ovary, and the optical density of AMH bands are expressed relative to β-actin. (B) Mean (+ SEM) AMH optical density for SD (filled bar) and LD (open bar) ovaries (n = 7 for each group); * denotes significant effect of photoperiod.
Serum AMH ELISA
Despite higher AMH protein expression in the ovaries of SD females, serum AMH concentration was significantly lower in SD than in LD females (t = 3.00; p = 0.01) (Fig. 3). The correlation (r = −0.353) between ovarian and serum AMH was not significant (p = 0.09). As with ovarian AMH, presence or absence of CLs in ovaries of LD females did not seem to overtly impact serum AMH concentration (LD with CL: 3.7 ± 0.6 ng/ml, n = 3; LD without CL: 3.4 ± 0.4 ng/ml, n = 3).
Figure 3.
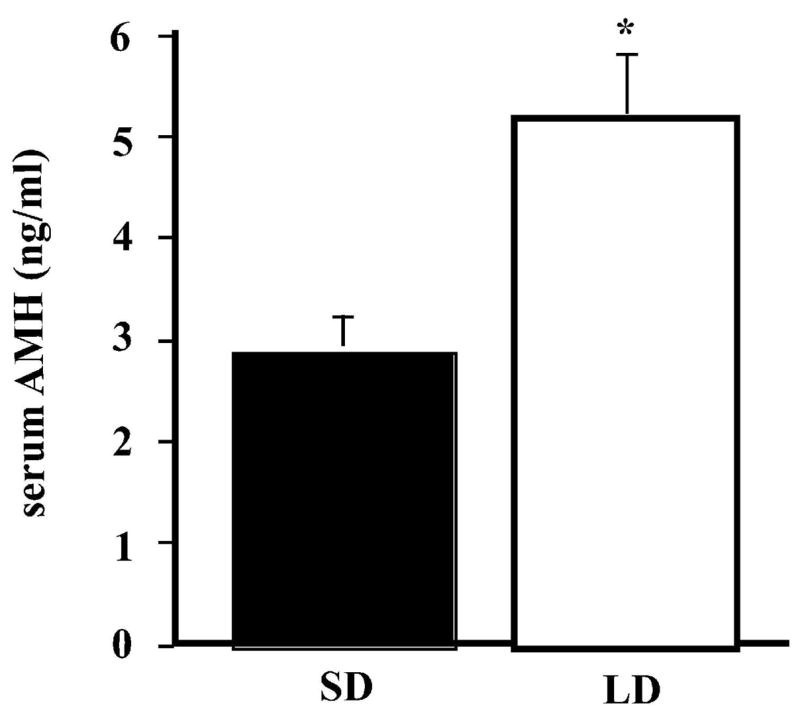
Mean (+ SEM) serum AMH concentration (ng/ml) in SD (filled bar) and LD (open bar) females (n = 7 for each group); * denotes significant effect of photoperiod.
Discussion
Siberian hamsters raised in SD demonstrate a profound inhibition of somatic and reproductive development (Ebling 1994, Place et al. 2004, Timonin et al. 2006), and the ovarian morphology of SD females is proving to be very interesting. Because AMH has been shown to inhibit primordial follicle activation (Durlinger et al. 1999, 2002a) and SD female hamsters had significantly more primordial follicles than age-matched LD hamsters (Place et al. 2004), we postulated that ovarian AMH might be modulated by photoperiod. The three-fold higher AMH levels in SD as compared to LD ovaries is an attractive mechanistic candidate for the preservation of primordial follicles and deceleration of reproductive aging in female hamsters raised in SD. However, the findings of the present study represent correlation and not causation. Nevertheless, these initial results are a good starting point for elucidating the means by which day length influences the numbers of primordial follicles and reproductive aging in P. sungorus (Place et al. 2004).
Lower AMH concentration in the serum of SD as compared to LD females suggests the actions of AMH on primordial follicle activation may be paracrine or autocrine in nature, as proposed by others (Ingraham et al. 2000, Durlinger et al. 2002, Knight & Glister 2006). The disparity between ovarian and serum AMH levels in LD and SD hamsters may reflect the variation in their ovarian morphology. For example, differences in the vascularization of SD and LD ovaries may explain the discrepancy. Ovaries from 10 wk-old SD females weighed significantly less than LD ovaries (Timonin et al. 2006, present study, Table 1) and thus SD ovaries may be less vascular. In fact, granulosa cells within ovarian follicles form an avascular layer (Irving-Rodgers & Rodgers 2000) and the AMH-expressing hypertrophied granulosa cells, which account for much of the SD ovary’s volume (Fig. 1C), appear to be luteinized but lack the increased vascularity that characterizes corpora lutea. Female hamsters raised in SD had more primordial follicles than age-matched LD females (Place et al. 2004, Timonin et al. 2006), and because Kevenaar et al. (2006) found circulating AMH to correlate with the size of the primordial follicle pool in mice, we expected serum AMH to be higher in SD than in LD females. SD hamsters showed an advantage over LD females in primordial follicle numbers at 13 and 26 weeks of age (Place et al. 2004), and a preliminary determination of the serum AMH concentration in animals from that study revealed lower AMH in SD at 13 wk of age, but higher AMH in SD at 26 wk of age (unpublished). We have interpreted these results with caution because the serum samples used had undergone a thaw-refreeze-thaw cycle before AMH determination. Nevertheless, it appears the relationship between the primordial follicle reserves and circulating AMH concentration in Siberian hamsters may be more complex than in mice, and day length may be a modulating factor. Differences in reproductive state may also contribute to the lower serum AMH levels in SD as compared to LD hamsters. Some years ago, serum AMH was reported to be barely detectable or extremely low in prepubertal women (Hudson et al. 1990, Lee & Donahoe 1993), but these investigators used an ELISA with limited sensitivity (0.5 ng/ml). The sensitivity of newer ELISA’s has improved (0.006 ng/ml) and a more recent study has reported appreciable levels of AMH in the serum of infants and young girls (Sir-Petermann et al. 2006). However, these same investigators measured higher levels in peripubertal girls (Crisosto et al. 2007), which suggests serum AMH is relatively low in females before puberty. Thus, lower serum AMH concentration in10 wk-old SD hamsters may simply reflect their pre-pubertal state, but still, the underlying etiology remains unknown.
Because we found LD ovaries had a comparable or greater number of the follicle types that express AMH most intensely (types 4 and 5) as compared to SD ovaries (Table 2), we suspect the greater AMH expression seen in SD ovaries by Western blot probably reflects the contribution made by the AMH-expressing luteinized granulosa cells. Alternatively, AMH expression may be up-regulated in granulosa cells that are affiliated with healthy follicles in SD ovaries. Why granulosa cells in SD ovaries persist and luteinize whilst they surround an atretic oocyte remains to be determined. A similar phenotype has been seen in mice deficient in growth differentiation factor-9 (GDF9), in that granulosa cells within primary follicles luteinized when the oocyte underwent atresia (Elvin et al. 1999). Those investigators did not assess AMH expression in the luteinized granulosa cells from GDF9 deficient mice, but the luteinized cells were positive for some luteal markers (e.g., LH receptor and P-450 side chain cleavage [P450scc]) as well as nonluteal markers (e.g., inhibin α and P-450 aromatase [P450arom]). We did not detect AMH expression in the Siberian hamster CL (Fig 1C), which means the luteinized granulosa cells in SD ovaries express at least one nonluteal marker. In contrast to the GDF9 deficient mouse, Kenny et al. (2002a) reported the luteinized granulosa cells from Siberian hamsters held in SD probably do not express the inhibin α-subunit. However, their figures (in situ hybridization) do not provide the histological details necessary to clearly distinguish cell and follicle types. Nevertheless, Kenny et al. (2002a) found significantly lower levels of inhibin α-subunit in ovaries from SD versus LD females.
The intense eosinophilia of the luteinized granulosa cells within hamster SD ovaries suggests they are active in steroidogenesis, and van den Hurk et al. (2002) reported especially strong enzyme activity for 3β-hydroxysteroid dehydrogenase (3β-HSD) in what they referred to as “hypertrophied granulosa cells of luteinized atretic follicles”. P450arom activity or expression has not been investigated in SD hamster ovaries, but van den Hurk et al. (2002) found serum estradiol (E2) concentration was significantly higher in SD than in LD female hamsters on postnatal days 28, 56, and 80. Whether luteinized granulosa cells account for the higher circulating E2 via P450arom activity remains to be determined. However, the higher serum E2 concentration reported for SD female hamsters (van den Hurk et al. 2002) seems paradoxical and warrants confirmation. The uterus, a highly estrogen sensitive organ, weighs significantly less in female hamsters raised in SD as compared to LD (Ebling 1994, Place et al. 2004, Timonin et al. 2006; present study – Table 1), and we have recently confirmed this finding (unpublished results) at all ages for which van den Hurk et al. (2002) reported significantly higher serum E2 levels in SD females. Moreover, Scotti et al. (2007) used the same commercial radioimmunoassay (RIA) kit as van den Hurk et al. (2002) to measure serum E2 in Siberian hamsters held in LD and SD, except Scotti et al. (2007) performed a diethyl ether extraction of steroids before proceeding with the RIA. Scotti et al. (2007) found no significant effect of photoperiod on serum E2 concentration, however their SD hamsters were adults that had been transferred from LD to SD, whereas van den Hurk et al. (2002) transferred hamsters from LD to SD at birth. Because E2 has been purported to both inhibit (Barrends et al. 1995, Balla et al. 2003) and stimulate (Ikeda et al. 2002) ovarian AMH expression, it will be important to determine if P450arom is expressed by luteinized granulosa cells and if hamsters raised in SD in fact have higher serum E2 concentrations than females reared in LD. Unfortunately, sample volume limitations precluded us from measuring serum E2 in the present study.
The circulating concentration of follicle stimulating hormone (FSH) is lower in female Siberian hamsters when raised in SD as compared to LD (Kenny et al. 2002a), and this may contribute to the differences in ovarian morphology and AMH expression. Bareends et al. (1995) noted a decrease in ovarian Amh expression following administration of human recombinant FSH to rats, and Balla et al. (2003) reported an up-regulation of AMH in ovarian granulosa cells in the follitropin receptor knockout (FORKO) mouse. Similar to SD hamsters, in which antral follicles are rare (present study) or absent (Place et al. 2004, Timonin et al. 2006), FORKO mice lack ovarian follicles that develop beyond the preantral stage. However, luteinized granulosa cells have not been reported in the ovaries of FORKO mice, and this may reflect differences in the hormonal milieu as compared to SD hamsters. Plasma LH was elevated in FORKO mice relative to wild-type and plasma estradiol was undetectable (Balla et al. (2003). Conversely, serum LH concentration has been reported to be much lower in SD than in LD female hamsters (Dodge & Badura, 2002), but E2 concentration does not appear to be consistently lower in SD as compared to LD hamsters (van den Hurk et al. 2002, Scotti et al. 2007; but see Moffatt-Blue et al. 2006). The similarities and differences in the ovarian phenotype of SD hamsters with that of GDF9 deficient and FORKO mice should help direct future investigations to determine the mechanisms/signals that underlie the luteinization of granulosa cells and the up-regulation of AMH in SD ovaries. Logical starting points will be to examine SD-ovary expression of GDF9 as well as GATA4, which mediates the inhibition of AMH via the FSH receptor (Tremblay & Viger 2001, Balla et al. 2003).
In addition to its inhibitory effects on primordial follicle activation (Durlinger et al. 1999, 2002a), AMH has also been shown to inhibit the growth of preantral and antral follicles in mice (Durlinger et al. 2001), by modulating the sensitivity of growing follicles to FSH (Visser et al. 2006). The higher AMH expression in SD hamster ovaries may explain the findings reported by Kenny et al. (2002b), whereby the ovarian response to an in vivo gonadotropin challenge (pregnant mare serum gonadotropin, PMSG) was blunted in juvenile SD hamsters relative to LD females. PMSG-induced follicular growth that was not as great in SD as compared to LD females, and SD females could not be induced to ovulate when an LH analog (human chorionic gonadotropin) was administered 48 h after PMSG. These results are intriguing in light of the recent report that AMH production by granulosa cells from women with polycystic ovary syndrome (PCOS) was significantly increased as compared to normal ovaries, and this may contribute to the anovulatory phenotype in PCOS (Pellatt et al. 2007).
In conclusion, the profound effect of photoperiod on reproductive function in female Siberian hamsters, and on their ovarian physiology in particular, suggests this species will be a valuable animal model for the study of ovarian follicle development. The activation of primordial follicles is still a poorly understood phenomenon, and the findings that SD rearing preserves the number of primordial follicles and enhances ovarian AMH expression in Phodopus sungorus adds further support for the critical role that AMH plays in follicular dynamics. Because we have the potential to dictate when juvenile SD females transition to the LD (mature) ovarian phenotype, simply by transferring them to a LD photoperiod, we may be able to better understand the conditions and signals in the ovary that modulate follicle activation and later stages of follicular development.
Acknowledgments
The authors would like to thank the staff of Laboratory Animal Services at Cornell University, and Jackie Belliveau in particular, for the exceptional care of our animals. We thank Jenifer Criuckshank and two anonymous referees for their helpful comments on the manuscript. The authors declare that there is no conflict of interest that would prejudice the impartiality of this scientific work. This study was supported by NIH grant HD-050358.
Footnotes
Publisher's Disclaimer: This is not the definitive version of record of this article. This manuscript has been accepted for publication in Reproduction, but the version presented here has not yet been copy edited, formatted or proofed. Consequently, the journal accepts no responsibility for any errors or omissions it may contain. The definitive version is now freely available at DOI: 10.1530/REP-07-0423.
References
- Adam CL, Moar KM, Logie TJ, Ross AW, Barrett P, Morgan PJ, Mercer JG. Photoperiod regulates growth, puberty and hypothalamic neuropeptide and receptor gene expression in female Siberian hamsters. Endocrinology. 2000;141:4349–4356. doi: 10.1210/endo.141.12.7807. [DOI] [PubMed] [Google Scholar]
- Baarends WM, Uilenbroek JT, Kramer P, Hoogerbrugge JW, van Leeuwen EC, Themmen AP, Grootegoed JA. Anti-Müllerian hormone and anti-müllerian hormone type II receptor messenger ribonucleic acid expression in rat ovaries during postnatal development, the estrous cycle, and gonadotropin-induced follicle growth. Endocrinology. 1995;136:4951–4962. doi: 10.1210/endo.136.11.7588229. [DOI] [PubMed] [Google Scholar]
- Balla A, Danilovich N, Yang Y, Sairam MR. Dynamics of Ovarian Development in the FORKO immature mouse: structural and functional implications for ovarian reserve. Biology of Reproduction. 2003;69:1281–1293. doi: 10.1095/biolreprod.103.015552. [DOI] [PubMed] [Google Scholar]
- Bath LE, Wallace WH, Shaw MP, Fitzpatrick C, Anderson RA. Depletion of ovarian reserve in young women after treatment for cancer in childhood: detection by anti-Müllerian hormone, inhibin B and ovarian ultrasound. Human Reproduction. 2003;18:2368–2374. doi: 10.1093/humrep/deg473. [DOI] [PubMed] [Google Scholar]
- Crisosto N, Codner E, Maliqueo M, Echiburu B, Sanchez F, Cassorla F, Sir-Petermann T. Anti-Mullerian hormone levels in peripubertal daughters of women with polycystic ovary syndrome. Journal of Clinical Endocrinology and Metabolism. 2007;92:2739–43. doi: 10.1210/jc.2007-0267. [DOI] [PubMed] [Google Scholar]
- Dodge JC, Badura LL. 5HT and 5HIAA dialysate levels within the arcuate nucleus of the hypothalamus: relationship with photoperiod-driven differences in serum prolactin and luteinizing hormone in the Siberian hamster. Brain Research. 2002;946:171–178. doi: 10.1016/s0006-8993(02)02874-3. [DOI] [PubMed] [Google Scholar]
- Durlinger AL, Kramer P, Karels B, de Jong FH, Uilenbroek JT, Grootegoed JA, Themmen AP. Control of primordial follicle recruitment by anti-Müllerian hormone in the mouse ovary. Endocrinology. 1999;140:5789–5796. doi: 10.1210/endo.140.12.7204. [DOI] [PubMed] [Google Scholar]
- Durlinger AL, Gruijters MJ, Kramer P, Karels B, Kumar TR, Matzuk MM, Rose UM, de Jong FH, Uilenbroek JT, Grootegoed JA, Themmen AP. Anti-Müllerian hormone attenuates the effects of FSH on follicle development in the mouse ovary. Endocrinology. 2001;142:4891–4899. doi: 10.1210/endo.142.11.8486. [DOI] [PubMed] [Google Scholar]
- Durlinger AL, Gruijters MJ, Kramer P, Karels B, Ingraham HA, Nachtigal MW, Uilenbroek JT, Grootegoed JA, Themmen AP. Anti-Müllerian hormone inhibits initiation of primordial follicle growth in the mouse ovary. Endocrinology. 2002a;143:1076–1084. doi: 10.1210/endo.143.3.8691. [DOI] [PubMed] [Google Scholar]
- Durlinger AL, Visser JA, Themmen AP. Regulation of ovarian function: the role of anti-Müllerian hormone. Reproduction. 2002b;124:601–609. doi: 10.1530/rep.0.1240601. [DOI] [PubMed] [Google Scholar]
- Ebling FJP. Photoperiodic differences during development in the dwarf hamsters Phodopus sungorus and Phodopus campbelli. General and Comparative Endocrinology. 1994;95:475–482. doi: 10.1006/gcen.1994.1147. [DOI] [PubMed] [Google Scholar]
- Elvin JA, Yan C, Wang P, Nishimori K, Matzuk MM. Molecular characterization of the follicle defects in the growth differentiation factor 9-deficient ovary. Molecular Endocrinology. 1999;13:1018–1034. doi: 10.1210/mend.13.6.0309. [DOI] [PubMed] [Google Scholar]
- Faddy MJ, Gosden RG. A model conforming the decline in follicle numbers to the age of menopause in women. Human Reproduction. 1996;11:1484–1486. doi: 10.1093/oxfordjournals.humrep.a019422. [DOI] [PubMed] [Google Scholar]
- Fortune JE. The early stages of follicular development: activation of primordial follicles and growth of preantral follicles. Animal Reproduction Science. 2003;78:135–163. doi: 10.1016/s0378-4320(03)00088-5. [DOI] [PubMed] [Google Scholar]
- Hirobe S, He WW, Lee MM, Donahoe PK. Müllerian inhibiting substance messenger ribonucleic acid expression in granulosa and Sertoli cells coincides with their mitotic activity. Endocrinology. 1992;131:854–862. doi: 10.1210/endo.131.2.1639028. [DOI] [PubMed] [Google Scholar]
- Hirshfield AN. Relationship between the supply of primordial follicles and the onset of follicular growth in rats. Biology of Reproduction. 1994;50:421–428. doi: 10.1095/biolreprod50.2.421. [DOI] [PubMed] [Google Scholar]
- Hudson PL, Dougas I, Donahoe PK, Cate RL, Epstein J, Pepinsky RB, MacLaughlin DT. An immunoassay to detect human mullerian inhibiting substance in males and females during normal development. Journal of Clinical Endocrinology and Metabolism. 1990;70:16–22. doi: 10.1210/jcem-70-1-16. [DOI] [PubMed] [Google Scholar]
- Ingraham HA, Hirokawa Y, Roberts LM, Mellon SH, McGee E, Nachtigal MW, Visser JA. Autocrine and paracrine Müllerian inhibiting substance hormone signaling in reproduction. Recent Progress in Hormone Research. 2000;55:53–67. [PubMed] [Google Scholar]
- Ikeda Y, Nagai A, Ikeda MA, Hayashi S. Increased expression of Müllerian-inhibiting substance correlates with inhibition of follicular growth in the developing ovary of rats treated with E2 benzoate. Endocrinology. 2002;143:304–312. doi: 10.1210/endo.143.1.8603. [DOI] [PubMed] [Google Scholar]
- Jost A. Recherches sur la differenciation sexuelle de l’embryon de lapin. Archives d’anatomie microscopique et de morphologie expérimentale. 1947;36:217–315. [Google Scholar]
- Kenny HA, Bernard DJ, Horton TH, Woodruff TK. Photoperiod-dependent regulation of inhibin in Siberian hamsters: I. Ovarian inhibin production and secretion. Journal of Endocrinology. 2002a;174:71–83. doi: 10.1677/joe.0.1740071. [DOI] [PubMed] [Google Scholar]
- Kenny HA, Bernard DJ, Horton TH, Woodruff TK. Photoperiod-dependent regulation of inhibin in Siberian hamsters: II. Regulation of inhibin production and secretion by pregnant mare serum gonadotropin. Journal of Endocrinology. 2002b;174:85–94. doi: 10.1677/joe.0.1740085. [DOI] [PubMed] [Google Scholar]
- Kevenaar ME, Meerasahib MF, Kramer P, van de Lang-Born BM, de Jong FH, Groome NP, Themmen AP, Visser JA. Serum AMH levels reflect the size of the primordial follicle pool in mice. Endocrinology. 2006;147:3228–3234. doi: 10.1210/en.2005-1588. [DOI] [PubMed] [Google Scholar]
- Knight PG, Glister C. TGF-beta superfamily members and ovarian follicle development. Reproduction. 2006;132:191–206. doi: 10.1530/rep.1.01074. [DOI] [PubMed] [Google Scholar]
- Lee MM, Donahoe PK. Mullerian inhibiting substance: a gonadal hormone with multiple functions. Endocrine Reviews. 1993;14:152–64. doi: 10.1210/edrv-14-2-152. [DOI] [PubMed] [Google Scholar]
- Liao J, Xu X, Wargovich MJ. Direct reprobing with anti-β-actin as an internal control for western blot analysis. Biotechniques. 2000;28:216–218. doi: 10.2144/00282bm05. [DOI] [PubMed] [Google Scholar]
- Moffatt-Blue CS, Sury JJ, Young KA. Short photoperiod-induced ovarian regression is mediated by apoptosis in Siberian hamsters (Phodopus sungorus) Reproduction. 2006;131:771–782. doi: 10.1530/rep.1.00870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen T, Peters H. Proposal for a classification of oocytes and follicles in the mouse ovary. Journal of Reproduction and Fertility. 1968;17:555–557. doi: 10.1530/jrf.0.0170555. [DOI] [PubMed] [Google Scholar]
- Pellatt L, Hanna L, Brincat M, Galea R, Brain H, Whitehead S, Mason H. Granulosa cell production of anti-Müllerian hormone is increased in polycystic ovaries. Journal of Clinical Endocrinology and Metabolism. 2007;92:240–255. doi: 10.1210/jc.2006-1582. [DOI] [PubMed] [Google Scholar]
- Place NJ, Tuthill CR, Schoomer EE, Tramontin AD, Zucker I. Short day lengths delay reproductive aging. Biology of Reproduction. 2004;71:987–992. doi: 10.1095/biolreprod.104.029900. [DOI] [PubMed] [Google Scholar]
- Scotti MA, Place NJ, Demas GE. Short-day increases in aggression are independent of circulating gonadal steroids in female Siberian hamsters (Phodopus sungorus) Hormones and Behavior. 2007;52:183–190. doi: 10.1016/j.yhbeh.2007.03.029. [DOI] [PubMed] [Google Scholar]
- Sir-Petermann T, Codner E, Maliqueo M, Echiburu B, Hitschfeld C, Crisosto N, Perez-Bravo F, Recabarren SE, Cassorla F. Increased anti-Mullerian hormone serum concentrations in prepubertal daughters of women with polycystic ovary syndrome. Journal of Clinical Endocrinology and Metabolism. 2006;91:3105–9. doi: 10.1210/jc.2005-2693. [DOI] [PubMed] [Google Scholar]
- Timonin ME, Place NJ, Wanderi E, Wynne-Edwards KE. Phodopus campbelli detect reduced photoperiod during development but, unlike Phodopus sungorus, retain functional reproductive physiology. Reproduction. 2006;132:661–670. doi: 10.1530/rep.1.00019. [DOI] [PubMed] [Google Scholar]
- Tremblay JJ, Viger RS. GATA factors differentially activate multiple gonadal promoters through conserved GATA regulatory elements. Endocrinology. 2001;142:977–986. doi: 10.1210/endo.142.3.7995. [DOI] [PubMed] [Google Scholar]
- Ueno S, Kuroda T, Maclaughlin DT, Ragin RC, Manganaro TF, Donahoe PK. Müllerian inhibiting substance in the adult rat ovary during various stages of the estrous cycle. Endocrinology. 1989;125:1060–1066. doi: 10.1210/endo-125-2-1060. [DOI] [PubMed] [Google Scholar]
- van den Hurk R, Dijkstra G, De Jong F. Enhanced serum oestrogen levels and highly steroidogenic, luteinized atretic follicles in the ovaries of the Djungarian hamster (Phodopus sungorus) kept under a short photoperiod from birth. European Journal of Endocrinology. 2002;147:701–710. doi: 10.1530/eje.0.1470701. [DOI] [PubMed] [Google Scholar]
- van Rooij IA, Broekmans FJ, Scheffer GJ, Looman CW, Habbema JD, de Jong FH, Fauser BJ, Themmen AP, te Velde ER. Serum antiMüllerian hormone levels best reflect the reproductive decline with age in normal women with proven fertility: a longitudinal study. Fertility and Sterility. 2005;83:979–987. doi: 10.1016/j.fertnstert.2004.11.029. [DOI] [PubMed] [Google Scholar]
- Visser JA, de Jong FH, Laven JS, Themmen AP. Anti-Müllerian hormone: a new marker for ovarian function. Reproduction. 2006;131:1–9. doi: 10.1530/rep.1.00529. [DOI] [PubMed] [Google Scholar]


