Abstract
We have recently described the development of a luminescent Mycobacterium paratuberculosis strain of bovine origin expressing the luxAB genes of Vibrio harveyi. With this luminescent isolate, fastidious and costly enumeration of CFU by plating them on agar can be replaced by easy and rapid luminometry. Here, we have reevaluated the effect of Slc11a1 (formerly Nramp1) polymorphism on susceptibility to M. paratuberculosis, using this luminometric method. A series of inbred mouse strains were infected intravenously with luminescent M. paratuberculosis S-23 and monitored for bacterial replication in spleen, liver, and lungs for 12 weeks. The results indicate that, as for Mycobacterium avium subsp. avium, innate resistance to infection is genetically controlled by Slc11a1. In BALB/c, congenic BALB.B10-H2b (BALB/c background; H-2b), C57BL/6, and beige C57BL/6bg/bg mice (all Slc11a1s), bacterial numbers in spleen and liver remained unchanged during the first 4 weeks of infection, whereas in DBA/2 and congenic BALB/c.DBA/2 (C.D2) mice (both Slc11a1r) and in (C57BL/6 × DBA/2)F1 mice (Slc11a1s/r), the bacterial numbers had decreased more than 10-fold at 4 weeks postinfection in both male and female mice. At later time points, additional differences in bacterial replication were observed between the susceptible mouse strains, particularly in the liver. Whereas bacterial numbers in the liver gradually decreased more than 100-fold in C57BL/6 mice between week 4 and week 12, bacterial numbers were stable in livers from BALB/c and beige C57BL/6bg/bg mice during this period. Mycobacterium-specific gamma interferon responses developed earlier and to a higher magnitude in C57BL/6 mice than in BALB/c mice and were lowest in resistant C.D2 mice.
The natural resistance of mice to the intracellular pathogens Leishmania donovani and Salmonella enterica serovar Typhimurium and to Mycobacterium bovis BCG vaccine is controlled by the natural resistance-associated macrophage protein 1 (NRAMP1), now called SLC11A1 (34). The Slc11a1 gene is expressed in late endosomes of macrophages derived from spleen and liver, in which it regulates antimicrobial activity (12). Several studies have demonstrated that the SLC11A1 protein is involved directly or indirectly in the maturation process of the phagosome by transport of bivalent cations (such as Fe2+, Mn2+, or Mg2+), but the mechanism by which parasite replication is blocked has not been completely elucidated (11, 20, 47).
The natural resistance of mice to nontuberculous mycobacterial infections caused by Mycobacterium simiae, Mycobacterium intracellulare, and Mycobacterium avium subsp. avium is also controlled by Slc11a1 (1, 27). The role of Slc11a1 in susceptibility to Mycobacterium paratuberculosis, the etiological agent of bovine paratuberculosis, or Johne's disease, is less clear and has been studied mostly in the context of Crohn's disease. The association between M. paratuberculosis and Crohn's disease has been questioned for a long time, but recent improvements in isolation and genomic techniques seem to suggest a stronger association of M. paratuberculosis as either a causative agent or an opportunistic infection of Crohn's disease patients (6, 7, 26). Three studies have examined Slc11a1 polymorphisms in patients with inflammatory bowel disease (15, 33, 38). From these reports, it can be concluded that the etiology of Crohn's disease is the result of a complex interplay of genetic, infectious, and immunologic factors, and (as for studies of AIDS patients with pulmonary M. avium complex infection) these observations suggest that Slc11a1 is one, but only one, determinant of genetic susceptibility (39).
Mice are generally considered to be resistant to M. paratuberculosis and unsuitable for the study of this intestinal pathogen of cattle, goats, sheep, and wild ruminants (13). Some authors have reported on genetic variations in the susceptibility of mice to M. paratuberculosis infection, but in these studies, bacterial replication was analyzed by measuring hepato- or splenomegaly (10, 40), not by actual enumeration of bacteria. The last technique is seriously hampered by the fact that M. paratuberculosis, a slowly growing mycobacterial species, requires 6 to 8 weeks of culture before colonies can be counted visually. Chiodini and Buergelt compared three susceptible Slc11a1s mouse strains (BALB/c, C57BL/6, and C57BL/10) using the LINDA strain isolated from a Crohn's disease patient (8). This study indicated that the reduction in bacterial burden was associated with the development of caseous necrotic lesions. Veazey et al. analyzed actual CFU counts in M. paratuberculosis-infected C57BL/6 and C3H mice, which express the susceptible and resistant Slc11a1 alleles, respectively, but which also differ at numerous other loci (43, 44).
We have recently reported on the construction of a luminescent M. paratuberculosis isolate that expresses the luxAB genes of Vibrio harveyi introduced by transformation with the shuttle plasmid pSMT1 (31). With this luminescent isolate, fastidious enumeration of CFU can be replaced by easy and inexpensive luminometry (31). Here, we have used this luminescent M. paratuberculosis isolate to reevaluate the role of Slc11a1 in the susceptibilities of a series of inbred mouse strains to intravenous M. paratuberculosis infection.
MATERIALS AND METHODS
Mice.
BALB/cOlaHsd (BALB/c), BALB.B10-H2b (BALB.B10), C57BL/6JOlaHsd (B6), and mutant C57BL/6OlaHsd-Lystbg/bg (B6bg/bg) beige mice (four strains expressing the susceptible Slc11a1s allele); DBA/2OlaHsd (DBA/2) and BALB/c.DBA/2 (C.D2) mice (two strains expressing the resistant Slc11a1r allele) (9); and heterozygous (C57BL/6 × DBA/2)F1 [(B6 × D2)F1] mice were bred at the Pasteur Institute Animal Facilities from breeding couples originally obtained from Harlan Netherlands (BALB/c, C57BL/6, DBA/2, and B6bg/bg), from The Netherlands Cancer Institute (BALB.B10), and from E. Skamene (McGill University, Montreal, Canada) (C.D2). All mice were 2 to 3 months old at the time of infection. B6bg/bg mice are spontaneous C57BL/6 mutants for the recessive gene bg (a lysosomal trafficking regulator). This mouse strain (H-2b) presents phenotypic manifestations resembling Chediak-Higashi syndrome in humans, and the mouse beige gene is actually a homolog of the human CHS1 gene (35). Beige mice have defects in blood clotting, reduced chemotactic and bactericidal activity of granulocytes, and abnormal giant lysosomal granules. These mice also have a severe deficiency in natural killer cell lytic activity. BALB.B10 mice are major histocompatibility complex congenic mice with an H-2b locus from C57BL/10 on a BALB/c background. C.D2-Slc11ar (BALB/c.DBA/2J) mice are BALB/c congenic mice that carry a 30-centimorgan segment of DBA/2 chromosome 1 containing the Slc11ar allele (28).
Luminescence assay.
The number of bioluminescent bacteria was determined using a bioluminescence assay with a Lumat LB 9507 luminometer (Berthold Technologies) and 1% n-decanal (Sigma) in ethanol as a substrate (41). In this assay, only live bacteria are enumerated, because emission of light is dependent on the presence of reduced flavin mononucleotide (FMNH2), a cofactor that is found only in living cells. For statistical analysis (two-way analysis of variance [ANOVA] with Bonferroni posttests), results obtained in relative light units (RLU) were converted to log10 values. The CFU/RLU ratio for exponentially growing axenic M. paratuberculosis cultures was determined to be 1.2 under our test conditions (5-second delay and 15-second integration time). It is important to note that RLU values are relative and not absolute light units, and the values depend on the sensitivity of the luminometer, the sample and substrate volumes, and the integration time used. However, in comparisons of CFU and RLU data, delta log10 values have been found to be nicely correlated (31, 41).
Infection of mice.
The luminescent bovine isolate M. paratuberculosis S-23 (31) was grown in Middlebrook 7H9 medium supplemented with oleic acid-albumin-dextrose-catalase, mycobactin J (Allied Laboratories Inc., Synbiotics Europe; 2 μg/ml), and hygromycin (100 μg/ml) to an optical density between 0.6 and 0.8. The bacteria were centrifuged for 30 min at 2,000 rpm and suspended in phosphate-buffered saline (PBS) to a concentration of 8.5 × 106 RLU/ml, and mice were infected intravenously in a lateral tail vein with 0.2 ml (1.7 × 106 RLU to 2 × 106 CFU) of bacteria.
Counting RLU in organs.
At day 1 and weeks 2, 4, 8, and 12 after infection, mice were killed by cervical dislocation; spleens, lungs, and livers were removed aseptically and homogenized by gentle disruption in a loosely fitting Dounce homogenizer. After red cell lysis (to minimize quenching), 1 ml of fresh organ homogenate was centrifuged and resuspended in 1 ml of PBS. The bacterial burden was evaluated by a luminescent assay as previously described (with 0.1 ml n-decanal as a substrate) (41). Background RLU values were determined in spleen, liver, and lungs of naïve BALB.B10 mice.
Counting CFU.
The number of CFU of luminescent M. paratuberculosis was determined by plating serial dilutions in PBS on Middlebrook 7H11-oleic acid-albumin-dextrose-catalase agar supplemented with mycobactin J and with (or without) hygromycin, in order to check for the presence of the pSMT1 plasmid. One hundred-microliter volumes of bacterial culture or organ homogenates were plated in duplicate. The petri dishes were sealed in plastic bags and incubated at 39°C for 8 weeks before the colonies were counted visually.
Bacterial antigens (Ags).
M. paratuberculosis ATCC 19698 was grown at 39°C for 4 weeks as a surface pellicle on synthetic Sauton medium supplemented with mycobactin J as described previously (30). The culture filtrate from M. paratuberculosis (CF-P) was separated from the bacteria; CF proteins were precipitated with ammonium sulfate and extensively dialyzed against PBS. The CF of M. bovis (AN5) (CF-B) was obtained from M. bovis cultures grown as surface pellicles for 2 weeks at 37°C on synthetic Sauton medium. Purified protein derivative from M. bovis Vallée (PPD-B) was kindly given to us by the late J. Nyabenda (WIV-Pasteur Institute Brussels). PPD was prepared from 6- to 8-week-old cultures of M. paratuberculosis strain ATCC 19698 (PPD-P). Recombinant Ag85B from M. paratuberculosis was prepared as described previously (30). Briefly, recombinant protein was expressed in Top-10F′ Escherichia coli transformed with a plasmid encoding Ag85B from M. paratuberculosis with six N-terminal histidines. Recombinant proteins were purified by affinity chromatography on an immobilized nickel-chelate (Ni-nitrilotriacetic acid) column.
Spleen cell gamma interferon (IFN-γ) production.
At 4, 8, and 12 weeks after infection, female mice were killed by cervical dislocation; their spleens were removed aseptically and homogenized by gentle disruption in a loosely fitting Dounce homogenizer. Spleens from three or four animals per group were analyzed individually. The spleen cells were washed and suspended to 4 × 106 leukocytes/ml in RPMI 1640 medium supplemented with 10% fetal calf serum, penicillin, streptomycin, 5 × 10−5 M 2-mercaptoethanol, and indomethacin (1 μg/ml; Sigma). The cells were cultured in a humidified CO2 incubator in round-bottom 96-well microplates. A volume of 180 μl of cells was added to 20 μl of the respective Ags. CF-B, CF-P, PPD-B, and PPD-P were used at final concentrations of 10 μg/ml and recombinant E. coli-derived 85B protein from M. paratuberculosis was used at 5 μg/ml in the presence of polymyxin B (5 ng/ml). Culture supernatants from three wells were collected and pooled after 72 h (the peak of cytokine activity) and stored at −20°C until they were tested.
IFN-γ assay.
IFN-γ activity was quantified on supernatants using a sandwich enzyme-linked immunosorbent assay with the coating antibody R4-6A2 and the biotinylated detection antibody XMG1.2 (both from Pharmingen). The detection limit was 10 pg/ml. The results were expressed as mean (± standard deviation) pg/ml, calculated from three or four mice per group tested individually.
For statistical analysis, two-way ANOVA with Bonferroni posttests was used to statistically evaluate differences in IFN-γ production from that of naïve mice, by time postinfection, between M. paratuberculosis Ag and M. bovis Ag or between mouse strains for Ag85B.
RESULTS
Replication of luminescent M. paratuberculosis S-23 in mice carrying the Slc11a1s (susceptible) or the Slc11a1r (resistant) allele.
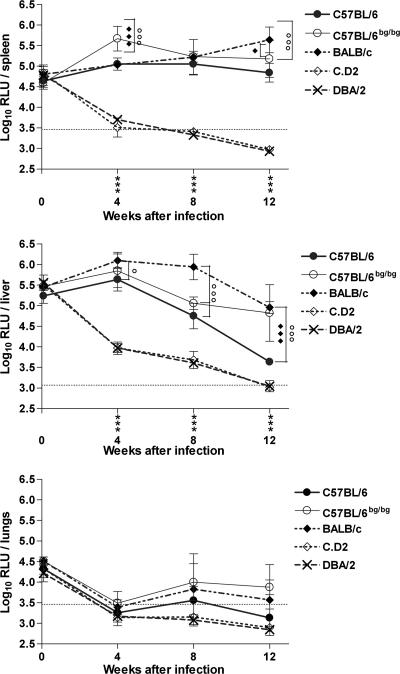
In order to determine whether susceptibility to M. paratuberculosis infection was influenced by the Slc11a1 gene, five inbred mouse strains were infected intravenously with 1.7 × 106 RLU of luminescent M. paratuberculosis S-23 and monitored for 12 weeks for replication in spleen, lungs, and liver. As shown in Fig. 1, DBA/2 and C.D2 congenic mice (both expressing the resistant Slc11a1r allele) eliminated M. paratuberculosis very rapidly (the values came down to the detection cutoff values 8 to 12 weeks after infection), whereas BALB/c, C57BL/6, and beige B6bg/bg mice expressing the susceptible Slc11a1s allele showed persistent infection. At week 4 postinfection, Slc11a1s mice showed 10- to 100-fold-higher RLU numbers in the liver and spleen than mice expressing the resistant Slc11a1r allele (P < 0.001). Monitoring infection for another 8 weeks demonstrated further differences between the three strains displaying the susceptible phenotype. In the spleens of BALB/c mice, bacterial numbers increased slightly until week 12, whereas in B6 and mutant B6bg/bg mice, splenic bacterial numbers remained stable. In the livers of B6 mice, bacterial numbers gradually declined by week 12 to levels almost comparable to the levels in resistant mice. In contrast, the bacterial loads were stable in livers from BALB/c mice. Interestingly, mutant beige B6bg/bg mice displayed the same hepatic phenotype as BALB/c mice, and the bacterial loads remained high in the livers of these mice, reported to have increased susceptibility to M. paratuberculosis (36). Finally, the bacterial burdens in the lungs decreased very rapidly in both Slc11a1s and Slca1a1r mice during the first month of infection. Interestingly, between week 4 and week 8, bacterial numbers increased again in the lungs of susceptible mice, particularly in beige B6bg/bg mice.
FIG. 1.
Susceptibilities of five inbred mouse strains to intravenous infection with luminescent M. paratuberculosis S-23. Mice were sacrificed on day 1 and 4, 8, and 12 weeks after infection, and the numbers of mycobacteria in spleen, liver, and lungs were determined by luminometry. The results are reported as log10 RLU/organ (mean ± standard deviation of three to six mice per group tested individually). The detection limits (dotted lines) were determined using organs from noninfected BALB.B10 mice. Statistical analyses were performed using two-way ANOVA with Bonferroni posttests. Comparison between susceptible and resistant mice: *, P < 0.05; **, P < 0.01; ***, P < 0.001. Comparison between BALB/c and C57BL/6 or C57BL/6bg/bg mice: ○, P < 0.05; ○○, P < 0.01; ○○○, P < 0.001. Comparison between C57BL/6 and C57BL/6bg/bg mice: ⧫, P < 0.05; ⧫ ⧫, P < 0.01; ⧫ ⧫ ⧫, P < 0.001.
In order to verify that these differences in resistance observed in luminometry corresponded to actual differences in numbers of bacteria, a limited set of organ homogenates was also plated on Middlebrook 7H11 agar with and without hygromycin (a resistance marker on the pSMT1 plasmid), and the actual number of CFU was determined. As shown in Table 1, the results obtained by luminometry closely matched those obtained by CFU plating. At 4 weeks after infection, 10-fold-higher CFU counts were detected in spleens and livers from susceptible B6 and BALB/c mice than in spleens and livers from resistant C.D2 mice. At week 12 postinfection, the differences were even more dramatic, and CFU counts in livers were about 100-fold lower in C.D2 than in BALB/c mice, confirming the difference observed in RLU (5.33 log10 versus 3.22 log10). At week 4, the CFU/RLU ratios in spleens were 1.9 and 2.95 in susceptible B6 and BALB/c mice and 6.6 in resistant C.D2 mice. In the liver, the respective CFU/RLU ratios were 12.3, 5.62, and 15.1, and in the lungs, they were 2.13, 6.4, and 32.3 at this early time point. These ratios reflected the observed resistance pattern, with high ratios being caused by impaired bacterial fitness (resulting in decreased metabolism and light emission of the bacteria). The CFU/RLU ratios at week 12 showed a similar trend, but this comparison at late time points has to be made with more caution because of some loss of the pSMT1 plasmid in vivo in susceptible animals (31). Thus, CFU counts in the presence or absence of the resistance marker hygromycin were comparable in the three organ homogenates of resistant C.D2 mice at week 12, whereas in livers from susceptible BALB/c mice, there was a 3.7-fold difference in this ratio. The luminescence assay of duplicate samples showed very reproducible results with a maximal variation of 10%, whereas results from CFU plating presented up to 25% intra-assay variation. Also, the range of detection for luminometry was much broader (between 103 and 107 RLU) than for CFU counting (10 to 100 CFU/petri dish), requiring multiple organ dilutions.
TABLE 1.
Evolution of M. paratuberculosis S-23 numbers in spleen, liver, and lungs as determined by luminometry and classical CFU counting
| Organ | Mouse | No. of bacteriaa
|
|||||
|---|---|---|---|---|---|---|---|
| 4 wk after infection
|
12 wk after infection
|
||||||
| Log10 RLU | Log10 CFU | Log10 CFU(h) | Log10 RLU | Log10 CFU | Log10 CFU(h) | ||
| Spleen | C57BL/6 | 5.29 ± 0.44 (5) | 5.56 ± 0.58 (5) | 5.39 ± 0.66 (5) | 4.59 ± 0.21 (5) | 5.80 ± 0.11 (5) | 5.47 ± 0.13 (5) |
| BALB/c | 5.16 ± 0.15 (4) | 5.63 ± 0.19 (4) | 5.66 ± 0.13 (4) | 5.23 ± 0.34 (4) | 6.20 ± 0.52 (3) | 6.02 ± 0.34 (3) | |
| C.D2 | 3.88 ± 0.10 (4) | 4.70 ± 0.17 (4) | 4.58 ± 0.19 (4) | 3.52 ± 0.12 (6) | 3.97 ± 0.38 (6) | 4.02 ± 0.28 (6) | |
| Liver | C57BL/6 | 5.59 ± 0.06 (5) | 6.68 ± 0.09 (5) | 6.52 ± 0.10 (5) | 3.65 ± 0.22 (5) | 5.48 ± 0.25 (5) | 5.24 ± 0.25 (5) |
| BALB/c | 5.87 ± 0.06 (4) | 6.62 ± 0.13 (4) | 6.38 ± 0.16 (4) | 5.33 ± 0.40 (3) | 7.07 ± 0.27 (3) | 6.50 ± 0.18 (3) | |
| C.D2 | 4.40 ± 0.14 (4) | 5.58 ± 0.19 (4) | 5.42 ± 0.24 (4) | 3.22 ± 0.10 (6) | 4.80 ± 0.19 (6) | 4.60 ± 0.34 (6) | |
| Lungs | C57BL/6 | 3.68 ± 0.17 (5) | 4.01 ± 0.36 (5) | 4.07 ± 0.48 (5) | 3.06 ± 0.07 (5) | 3.76 ± 0.63 (5) | 3.34 ± 0.51 (5) |
| BALB/c | 3.34 ± 0.02 (4) | 4.15 ± 0.66 (4) | 4.06 ± 0.70 (4) | 3.42 ± 0.71 (3) | 5.00 ± 0.62 (3) | 4.59 ± 0.73 (3) | |
| C.D2 | 2.90 ± 0.02 (4) | 4.41 ± 0.71 (4) | 4.26 ± 0.73 (4) | 2.95 ± 0.17 (6) | 2.85 ± 0.40 (6) | 2.89 ± 0.46 (6) | |
Organs from individual infected mice were homogenized, and the numbers of bacteria/organ were determined by luminometry or by plating the bacteria on Middlebrook 7H11 agar with [CFU(h)] or without (CFU) hygromycin. The data represent mean (± standard deviation) values for three to six mice (numbers are given in parentheses).
Thus, using both luminometry and CFU plating, we demonstrated that susceptibility to intravenous M. paratuberculosis infection is influenced by the mouse genotype. Moreover, results in C.D2 mice indicated that the Slc11a1 gene is very likely responsible for the resistant phenotype, although the role of another gene on the 30-centimorgan segment of DBA/2 chromosome 1 cannot be formally excluded.
Replication of luminescent M. paratuberculosis S-23 in male and female BALB.B10 mice and (C57BL/6 × DBA/2)F1 mice.
As shown in Table 2, major histocompatibility complex congenic BALB.B10 mice (H-2b haplotype on a BALB/c background) displayed the susceptible phenotype, with stable RLU counts in spleen between day 1 and week 4 postinfection and increased RLU counts in liver during that same period. Heterozygous (B6 × D2)F1 mice demonstrated a dramatic reduction in bacterial numbers (a 20- to 60-fold decrease, depending on the organ, between day 1 and week 4), indicating that the Slc11a1r resistant allele was dominant in its effect on susceptibility to M. paratuberculosis (Table 2). This dramatic reduction in RLU counts in resistant mice was observed as early as 2 weeks postinfection, highlighting the role of innate immunity in this resistance. Since Johne's disease in cattle does not show any preference for cows or bulls (25), we also compared genetic susceptibilities to luminescent M. paratuberculosis in male and female mice. There was no statistical difference in bacterial replication between male and female mice, either in resistant (B6 × D2)F1 or in susceptible BALB.B10 mice (Table 2) (P > 0.05). Susceptibilities were also similar in male and female B6 and BALB/c mice with the susceptible phenotype and in DBA/2 mice with the resistant phenotype (data not shown).
TABLE 2.
Replication of luminescent M. paratuberculosis S-23 in male and female BALB.B10 and (B6 × D2)F1 mice
| Organ | Time after infectionb | Colonizationa
|
|||
|---|---|---|---|---|---|
| BALB.B10
|
(B6 × D2)F1
|
||||
| M | F | M | F | ||
| Spleen | d1 | 5.09 ± 0.13 (5) | 4.84 ± 0.18 (4) | 5.25 ± 0.14 (3) | 5.44 ± 0.08 (5) |
| w2 | ND | 4.65 ± 0.38 (4) | 4.32 ± 0.09 (3) | 4.27 ± 0.09 (3) | |
| w4 | 5.01 ± 0.13 (5) | 5.03 ± 0.12 (5) | 3.83 ± 0.07 (5) | 3.81 ± 0.07 (5) | |
| w8 | 4.94 ± 0.23 (6) | 5.08 ± 0.30 (4) | 3.29 ± 0.06 (5) | 3.46 ± 0.17 (5) | |
| w12 | 4.81 ± 0.20 (4) | 4.80 ± 0.38 (6) | 3.64 ± 0.14 (6) | 3.54 ± 0.11 (7) | |
| Liver | d1 | 5.43 ± 0.14 (5) | 5.51 ± 0.21 (4) | 5.57 ± 0.26 (3) | 5.84 ± 0.08 (5) |
| w2 | ND | 5.09 ± 0.12 (4) | 3.98 ± 0.13 (3) | 4.03 ± 0.10 (3) | |
| w4 | 5.95 ± 0.04 (5) | 6.09 ± 0.05 (5) | 4.23 ± 0.08 (5) | 4.24 ± 0.24 (5) | |
| w8 | 5.55 ± 0.22 (6) | 5.61 ± 0.45 (4) | 3.40 ± 0.11 (5) | 3.39 ± 0.11 (5) | |
| w12 | 5.34 ± 0.55 (4) | 4.82 ± 0.58 (6) | 3.26 ± 0.07 (6) | 3.20 ± 0.03 (6) | |
| Lungs | d1 | 4.80 ± 0.17 (5) | 4.99 ± 0.40 (4) | 4.48 ± 0.18 (3) | 4.67 ± 0.20 (5) |
| w2 | ND | 3.81 ± 0.10 (4) | 3.27 ± 0.03 (3) | 3.57 ± 0.16 (3) | |
| w4 | 3.26 ± 0.39 (5) | 3.07 ± 0.15 (5) | 2.92 ± 0.04 (5) | 2.93 ± 0.09 (5) | |
| w8 | 3.82 ± 0.75 (6) | 4.05 ± 0.81 (4) | 3.01 ± 0.10 (5) | 2.95 ± 0.14 (5) | |
| w12 | 3.52 ± 0.69 (4) | 3.55 ± 0.73 (6) | 2.92 ± 0.07 (6) | 2.99 ± 0.20 (6) | |
Colonization levels of M. paratuberculosis S-23 in male (M) and female (F) BALB.B10 and (B6 × D2)F1 mice in spleen, liver, and lungs, as quantified on day 1 and 2, 4, 8 and 12 weeks after intravenous infection. The data represent mean log10 RLU±standard deviation from three to six mice (numbers are given in parentheses). ND, not determined.
d, day; w, week.
Mycobacterium-specific cell-mediated immune response following experimental M. paratuberculosis infection in susceptible and resistant mice.
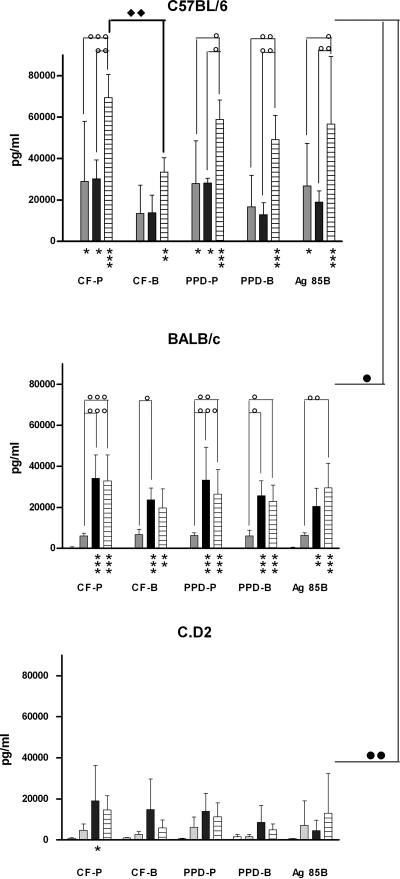
In order to analyze whether the cell-mediated immune response could be correlated with differences in susceptibility, production of the Th1-type cytokine IFN-γ was analyzed in spleen cell cultures from two M. paratuberculosis-infected susceptible mouse strains (B6 and BALB/c) and from one M. paratuberculosis-resistant strain (C.D2). As shown in Fig. 2, very strong mycobacterium-specific IFN-γ production could be detected in B6 mice as early as 1 month after infection in response to CF and PPD from M. paratuberculosis (about 30,000 pg/ml; P < 0.05). M. paratuberculosis-specific responses were higher than responses against CF and PPD from M. bovis, with statistical significance at 12 weeks postinfection (P < 0.01). Confirming previous findings (30), IFN-γ responses induced with recombinant Ag85B from M. paratuberculosis were also very high in B6 mice at 4 weeks postinfection (P < 0.05). As was also observed after intravenous M. bovis BCG vaccination (17), IFN-γ responses in BALB/c mice were about fivefold lower than in B6 mice (about 6,000 pg/ml) at week 4 postinfection and reached statistical significance at 12 weeks postinfection (P < 0.05 and P < 0.01, respectively). In B6 mice, IFN-γ levels did not change between week 4 and week 8 but increased again at week 12 up to maximal levels of between 60,000 and 70,000 pg/ml (P < 0.05). In BALB/c mice, IFN-γ responses reached a plateau between weeks 8 and 12 (maximal levels, around 30,000 pg/ml; P < 0.05). Differences between M. paratuberculosis-specific and M. bovis-specific responses remained weak in infected BALB/c mice. Finally, C.D2 mice demonstrated the lowest (albeit still substantial) IFN-γ response of the three mouse strains tested, and this was probably a reflection of the lack of M. paratuberculosis replication in the mouse strain. Maximal levels of about 10,000 pg/ml could be detected following stimulation with CF-P at 8 weeks postinfection (P < 0.05). IFN-γ responses by unstimulated cells and by stimulated cells from naïve, uninfected mice were low (between 100 and 1,000 pg/ml) and were slightly higher in C.D2 mice than in BALB/c and C57BL/6 mice.
FIG. 2.
Mycobacterium-specific IFN-γ secretion by splenocytes from M. paratuberculosis-infected C57BL/6, BALB/c, and C.D2 mice. IFN-γ production was measured in spleen cell culture supernatants from C57BL/6, BALB/c, and C.D2 mice before and 4 weeks (gray bars), 8 weeks (black bars), and 12 weeks (hatched bars) after infection with M. paratuberculosis S-23. The cells were stimulated for 72 h with CF-P or CF-B (10 μg/ml), PPD-P or PPD-B (10 μg/ml), or recombinant Ag85B (5 μg/ml) from M. paratuberculosis. Shown are means plus standard deviations of three or four mice tested individually. Statistical analyses were performed using two-way ANOVA with Bonferroni posttests. Comparison to naïve mice: *, P < 0.05; **, P < 0.01; ***, P < 0.001. Comparison to previous time point: ○, P < 0.05; ○○, P < 0.01; ○○○, P < 0.001. Comparison between M. paratuberculosis Ag and M. bovis Ag: ⧫, P < 0.05. Ag85B-specific responses compared between mouse strains: •, P < 0.05; ••, P < 0.01.
DISCUSSION
Although some genetic variations in susceptibility to M. paratuberculosis have been reported in cattle and red deer (14, 21), the role of Slc11a1 polymorphisms in Johne's disease is not very clear. In sheep, a large study of two fine-wool Merino flocks highly infected with M. paratuberculosis demonstrated associations of particular polymorphisms in the gene with susceptibility or resistance to infection (29). In other animal species, the situation seems more controversial. Here, we have demonstrated by a luminometric method that Slc11a1 polymorphism exerts a strong genetic influence on the innate susceptibility of mice to intravenous infection with M. paratuberculosis S-23, as indicated by a clear difference in bacterial replication in the spleen and liver between Slc11a1s and Slc11a1r mice. To our knowledge, this is the first time that the C.D2 congenic mouse strain, expressing the resistant Slc11a1r allele of DBA/2 origin on a BALB/c background, was used in an M. paratuberculosis study. It is clear that the intravenous infection route used in these experiments is not the natural route used by this enteric pathogen, and oral-infection experiments are needed to definitively demonstrate the role of Slc11a1 polymorphism in innate resistance to M. paratuberculosis.
NRAMP1, now called SLC11A1, was identified as a major, innate resistance component of host antimicrobial activity against a number of intracellular pathogens. This integral membrane protein, present in both prokaryotes and eukaryotes, is highly conserved, suggesting that it plays a basic physiological role, as proven by its conservation throughout evolution. In mice, this gene presents two allelic forms, one encoding an aspartic acid at position 169 (Slc11a1s) and the other encoding glycine at this position (Slc11a1r), which confer, respectively, susceptibility or resistance to infection by these pathogens (45). It is still not completely elucidated how SLC11A1 controls the replication of intracellular parasites. Some studies have suggested a direct involvement in the transport of iron or other bivalent cations, such as Mn2+ and Mg2+ (20). Induction of different degrees of iron overload by in vivo administration of iron-dextran in M. avium-infected mice seems to indicate that SLC11A1 contributes to macrophage antimicrobial function by excluding Fe2+ (essential for the pathogen) from the phagosomal vacuole through H+-coupled transport (11). Also, in vitro studies with M. avium showed that the addition of small quantities of iron to resident macrophages from Slc11a1r mice could stimulate antimicrobial activity through generation of hydroxyl radicals and stabilization of SLC11A1 mRNA (47).
The murine Slc11a1 gene is expressed in macrophages from spleen and liver but not from lungs (46). This could explain why in our experiments M. paratuberculosis infection was controlled to the same extent in Slc11a1s and Slc11a1r lungs during the first 4 weeks after intravenous infection. Bacteria were very rapidly eliminated from the lungs of both susceptible and resistant mice, indicating that Slc11a1-independent mechanisms are involved in the antimycobacterial defense of pulmonary macrophages. Interestingly, a study of women suffering from M. avium-M. intracellulare pulmonary disease also failed to find a correlation with Slc11a1 polymorphisms (16).
Slc11a1 polymorphism does not play a role in the susceptibility of mice (3, 23), red deer (21), or cattle (3, 4) to virulent M. bovis or M. tuberculosis, although in humans, certain Slc11a1 alleles are risk factors for tuberculosis (4, 5, 22), possibly through regulation of interleukin-10 production (2). The situation is complex, and the effect of Slc11a1 polymorphism on tuberculosis susceptibility seems to be restricted to particular ethnic groups, e.g., Asian subjects (19), and the association is sex and age dependent and restricted to females and the young age group (below 65 years) (18). Recently, a genetic polymorphism in the human Slc11a1 gene was found to be associated with susceptibility to another mycobacterial pathogen for humans, i.e., Mycobacterium ulcerans, which causes Buruli ulcer, a necrotizing skin disease particularly affecting children in Central and West Africa (37).
Whereas initial resistance to M. paratuberculosis was clearly controlled by Slc11a1, at later stages, additional factors influence bacterial replication in mice expressing the susceptible Slc11a1s allele. Thus, BALB/c mice controlled infection less efficiently than B6 mice in spleen, in lungs, and particularly in liver. Differences in the magnitudes of the early mycobacterium-specific Th1 (IFN-γ) response observed in these two mouse strains in the spleen may play a role, but certainly, other factors are also involved. Indeed, Ag-specific IFN-γ production was significantly lower in resistant C.D2 mice than in susceptible BALB/c and B6 mice. Also, beige B6bg/bg mice (presenting macrophage lysosomal defects and deficient lytic natural killer cell activity) were more susceptible than mice of the parental B6 strain, particularly with respect to control of the infection in the liver and lungs, although acquired immunity levels, reflected by mycobacterium-specific spleen cell IFN-γ responses, were comparable in B6 mice and in these mutant B6bg/bg mice (30). This suggests that attraction of neutrophils and natural killer cells to the liver could be an important control mechanism following intravenous M. paratuberculosis infection, as suggested by Saunders and Cheers for beige mice infected with M. avium (32). In this respect, it is interesting to note that mutations in nucleotide oligomerization domain 2 (Nod2), involved in signaling of proinflammatory chemokines, have been reported in 15% of patients suffering from Crohn's disease (24). A defect in neutrophil recruitment caused by a decreased interleukin-8 response to muramyl dipeptide has also been described in Crohn's disease (42). Clearly, more work is needed to characterize the precise immune mechanisms involved at later stages of M. paratuberculosis infection in mice, particularly with respect to proinflammatory chemokine production.
Acknowledgments
This work was partially supported by grants from the FWO-Vlaanderen (G.0376.05). V. Rosseels is supported by the Belgian Science Policy (Ylieff). V. Roupie is a FRIA bursary recipient. We also acknowledge support from the USDA Animal Health Project (NEB 14-108 to R.G.B.).
We are grateful to F. Jurion and P.-Y. Adnet for excellent technical assistance. We also thank E. Skamene (McGill University, Montreal, Canada) for giving us breeding couples of the congenic C.D2 mouse strain. Finally, we thank Erik Jongert (WIV-Pasteur Institute) for helping us with the statistical analyses.
Editor: J. L. Flynn
Footnotes
Published ahead of print on 19 February 2008.
REFERENCES
- 1.Appelberg, R., and A. M. Sarmento. 1990. The role of macrophage activation and of Bcg-encoded macrophage function(s) in the control of Mycobacterium avium infection in mice. Clin. Exp. Immunol. 80324-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Awomoyi, A., A. Marchant, J. Howson, K. McAdam, J. Blackwell, and M. Newport. 2002. Interleukin-10, polymorphism in SLC11A1 (formerly NRAMP1), and susceptibility to tuberculosis. J. Infect. Dis. 1861808-1814. [DOI] [PubMed] [Google Scholar]
- 3.Barthel, R., J. A. Piedrahita, D. N. McMurray, J. Payeur, D. Baca, F. Suarez Guemes, V. S. Perumaalla, T. A. Ficht, J. W. Templeton, and L. G. Adams. 2000. Pathologic findings and association of Mycobacterium bovis infection with the bovine NRAMP1 gene in cattle from herds with naturally occurring tuberculosis. Am. J. Vet. Res. 611140-1144. [DOI] [PubMed] [Google Scholar]
- 4.Bellamy, R. 2003. Susceptibility to mycobacterial infections: the importance of host genetics. Genes Immun. 44-11. [DOI] [PubMed] [Google Scholar]
- 5.Bellamy, R., N. Beyers, K. McAdam, C. Ruwende, R. Gie, P. Samaai, D. Bester, M. Meyer, T. Corrah, M. Collin, D. Camidge, D. Wilkinson, E. Hoal-Van Helden, H. Whittle, W. Amos, P. van Helden, and A. Hill. 2000. Genetic susceptibility to tuberculosis in Africans: a genome-wide scan. Proc. Natl. Acad. Sci. USA 978005-8009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bull, T., E. McMinn, K. Sidi-Boumedine, A. Skull, D. Durkin, P. Neild, G. Rhodes, R. Pickup, and J. Hermon-Taylor. 2003. Detection and verification of Mycobacterium avium subsp. paratuberculosis in fresh ileocolonic mucosal biopsy specimens from individuals with and without Crohn's disease. J. Clin. Microbiol. 412915-2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chacon, L., E. Bermudez, and R. G. Barletta. 2004. Johne's disease, inflammatory bowel disease and Mycobacterium paratuberculosis. Annu. Rev. Microbiol. 58329-363. [DOI] [PubMed] [Google Scholar]
- 8.Chiodini, R. J., and C. D. Buergelt. 1993. Susceptibility of BALB/c, C57/B6 and C57/B10 mice to infection with Mycobacterium paratuberculosis. J. Comp. Pathol. 109309-319. [DOI] [PubMed] [Google Scholar]
- 9.Denis, M., A. Forget, M. Pelletier, R. Turcotte, and E. Skamene. 1986. Control of the Bcg gene of early resistance in mice to infections with BCG substrains and atypical mycobacteria. Clin. Exp. Immunol. 63517-525. [PMC free article] [PubMed] [Google Scholar]
- 10.Frelier, P. F., J. W. Templeton, M. Estes, H. W. Whitford, and R. D. Kienle. 1990. Genetic regulation of Mycobacterium paratuberculosis infection in recombinant inbred mice. Vet. Pathol. 27362-364. [DOI] [PubMed] [Google Scholar]
- 11.Gomes, M. S., and R. Appelberg. 1998. Evidence for a link between iron metabolism and Nramp1 gene function in innate resistance against Mycobacterium avium. Immunology 95165-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gruenheid, S., E. Pinner, M. Desjardins, and P. Gros. 1997. Natural resistance to infection with intracellular pathogens: the Nramp1 protein is recruited to the membrane of the phagosome. J. Exp. Med. 185717-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris, N. B., and R. G. Barletta. 2001. Mycobacterium avium subsp. paratuberculosis in veterinary medicine. Clin. Microbiol. Rev. 14489-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hines, M. E., II, J. R. Stabel, R. W. Sweeney, F. Griffin, A. M. Talaat, D. Bakker, G. Benedictus, W. C. Davis, G. W. de Lisle, I. A. Gardner, R. A. Juste, V. Kapur, A. Koets, J. McNair, G. Pruitt, and R. H. Whitlock. 2007. Experimental challenge models for Johne's disease: a review and proposed international guidelines. Vet. Microbiol. 122197-222. [DOI] [PubMed] [Google Scholar]
- 15.Hofmeister, A., H. L. Neibergs, R. M. Pokorny, and S. Galandiuk. 1997. The natural resistance-associated macrophage protein gene is associated with Crohn's disease. Surgery 122173-178. [DOI] [PubMed] [Google Scholar]
- 16.Huang, J. H., P. J. Oefner, V. Adi, K. Ratnam, S. J. Ruoss, E. Trako, and P. N. Kao. 1998. Analyses of the NRAMP1 and IFNγR1 genes in women with Mycobacterium avium-intracellulare pulmonary disease. Am. J. Respir. Crit. Care Med. 157377-381. [DOI] [PubMed] [Google Scholar]
- 17.Huygen, K., D. Abramowicz, P. Vandenbussche, F. Jacobs, J. De Bruyn, A. Kentos, A. Drowart, J.-P. Van Vooren, and M. Goldman. 1992. Spleen cell cytokine secretion in Mycobacterium bovis BCG-infected mice. Infect. Immun. 602880-2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leung, K. H., S. P. Yip, W. S. Wong, L. S. Yiu, K. K. Chan, W. M. Lai, E. Y. Chow, C. K. Lin, W. C. Yam, and K. S. Chan. 2006. Sex- and age-dependent association of SLC11A1 polymorphism with tuberculosis in Chinese: a case control study. BMC Infect. Dis. 719-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li, H. T., T. T. Zhang, Y. Q. Zhou, Q. H. Huang, and J. Huang. 2006. SLC11A1 (formerly NRAMP1) gene polymporphism and tuberculosis susceptibility: a meta-analysis. Int. J. Tuberc. Lung Dis. 103-12. [PubMed] [Google Scholar]
- 20.Mackenzie, B., and M. A. Hediger. 2004. SLC11 family of H+-coupled metal-ion transporters NRAMP1 and DMT1. Eur. J. Physiol. 447571-579. [DOI] [PubMed] [Google Scholar]
- 21.Mackintosh, C. G., T. Qureshi, K. Waldrup, R. E. Labes, K. G. Dodds, and J. F. Griffin. 2000. Genetic resistance to experimental infection with Mycobacterium bovis in red deer (Cervus elaphus). Infect. Immun. 681620-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malik, S., L. Abel, H. Tooker, A. Poon, L. Simkin, M. Girard, G. Adams, J. Starke, K. Smith, E. Graviss, J. Musser, and E. Schurr. 2005. Alleles of the NRAMP1 gene are risk factors for pediatric tuberculosis disease. Proc. Natl. Acad. Sci. USA 10212183-12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Medina, E., and R. J. North. 1996. Evidence inconsistent with a role for the Bcg gene (Nramp1) in resistance to infection with Mycobacterium tuberculosis. J. Exp. Med. 1831045-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meinzer, U., and J.-P. Hugot. 2005. Nod2 and Crohn's disease: many connected highways. Lancet 3651752-1754. [DOI] [PubMed] [Google Scholar]
- 25.Merkal, R. S., D. L. Whipple, J. M. Sacks, and G. R. Snyder. 1987. Prevalence of Mycobacterium paratuberculosis in ileocecal lymph nodes of cattle culled in the United States. J. Am. Vet. Med. Assoc. 190676-680. [PubMed] [Google Scholar]
- 26.Naser, S. A., G. Ghobrial, C. Romero, and J. F. Valentine. 2004. Culture of Mycobacterium avium subsp. paratuberculosis from the blood of patients with Crohn's disease. Lancet 3641039-1044. [DOI] [PubMed] [Google Scholar]
- 27.Orme, I. M., R. W. Stokes, and F. M. Collins. 1986. Genetic control of natural resistance to nontuberculous mycobacterial infections in mice. Infect. Immun. 5456-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Potter, M., A. D. O'Brien, E. Skamene, P. Gros, A. Forget, P. A. Kongshavn, and J. S. Wax. 1983. A BALB/c congenic strain of mice that carries a genetic locus (Ityr) controlling resistance to intracellular parasites. Infect. Immun. 401234-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reddacliff, L. A., K. Beh, H. McGregor, and R. J. Whittington. 2005. A preliminary study of possible genetic influences on the susceptibility of sheep to Johne's disease. Aust. Vet. J. 83435-441. [DOI] [PubMed] [Google Scholar]
- 30.Rosseels, V., S. Marché, V. Roupie, M. Govaerts, J. Godfroid, K. Walravens, and K. Huygen. 2006. Members of the 30- to 32-kDa mycolyl transferase family (Ag85) from culture filtrate of Mycobacterium avium subsp. paratuberculosis are immunodominant Th1-type antigens recognized early upon infection in mice and cattle. Infect. Immun. 74202-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosseels, V., V. Roupie, D. Zinniel, R. G. Barletta, and K. Huygen. 2006. Development of luminescent M. avium subsp. paratuberculosis for rapid screening of vaccine candidates in mice. Infect. Immun. 743684-3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saunders, B. M., and C. Cheers. 1996. Intranasal infection of beige mice with Mycobacterium avium complex: role of neutrophils and natural killer cells. Infect. Immun. 644236-4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sechi, L. A., M. Gazouli, L. E. Sieswerda, P. Molicotti, N. Ahmed, J. Ikonomopoulos, A. M. Scanu, D. Paccagnini, and S. Zanetti. 2006. Relationship between Crohn's disease, infection with Mycobacterium avium subsp. paratuberculosis and SLC11A1 gene polymorphisms in Sardinian patients. World J. Gastroenterol. 287161-7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Skamene, E., E. Schurr, and P. Gros. 1998. Infection genomics: Nramp1 as a major determinant of natural resistance to intracellular infections. Annu. Rev. Med. 49275-287. [DOI] [PubMed] [Google Scholar]
- 35.Spritz, R. A. 1998. Genetic defects in Chediak-Higashi syndrome and the beige mouse. J. Clin. Immunol. 1897-105. [DOI] [PubMed] [Google Scholar]
- 36.Stabel, J. R., J. P. Goff, D. L. Whipple, M. R. Ackermann, and T. A. Reinhardt. 1996. Low calcium diet and 1,25-dihydroxyvitamin D(3) infusion modulate immune responses during Mycobacterium paratuberculosis infection in beige mice. Vet. Immunol. Immunopathol. 50127-143. [DOI] [PubMed] [Google Scholar]
- 37.Stienstra, Y., T. S. van der Werf, E. Oosterom, I. M. Nolte, W. T. A. van der Graaf, S. Etuaful, P. I. Raghunathan, E. A. S. Whitney, E. O. Ampadu, K. Asamoa, E. Y. Klutse, G. J. te Meerman, J. W. Tappero, D. A. Ashford, and G. van der Steege. 2006. Susceptibility to Buruli ulcer is associated with the SLC11A1 (NRAMP1) D543N polymorphism. Genes Immun. 7185-189. [DOI] [PubMed] [Google Scholar]
- 38.Stokkers, P. C. F., K. de Heer, A. C. Leegwater, P. H. Reitsma, G. N. J. Tytgat, and S. J. H. van Deventer. 1999. Inflammatory bowel disease and the genes for the natural resistance-associated macrophage protein-1 and the interferon-γ receptor 1. Int. J. Colorect. Dis. 1413-17. [DOI] [PubMed] [Google Scholar]
- 39.Tanaka, G., J. Shojima, I. Matsushita, H. Nagai, A. Kurashima, K. Nakata, E. Toyota, N. Kobayashi, K. Kudo, and N. Keicho. 2007. Pulmonary Mycobacterium avium complex infection: association with NRAMP1 polymorphisms. Eur. Respir. J. 301376-1382. [DOI] [PubMed] [Google Scholar]
- 40.Tanaka, S., M. Sato, T. Taniguchi, and Y. Yokomizo. 1994. Histopathological and morphometrical comparison of granulomatous lesions in BALB/c and C3H/HeJ mice inoculated with Mycobacterium paratuberculosis. J. Comp. Pathol. 410381-388. [DOI] [PubMed] [Google Scholar]
- 41.Tanghe, A., S. D'Souza, V. Rosseels, O. Denis, T. H. M. Ottenhoff, W. Dalemans, C. Wheeler, and K. Huygen. 2001. Improved immunogenicity and protective efficacy of a tuberculosis DNA vaccine encoding Ag85 by protein boosting. Infect. Immun. 693041-3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Heel, D. A., S. Ghosh, M. Butler, K. A. Hunt, A. M. Lundberg, T. Ahmad, D. P. B. McGovern, C. Onnie, K. Negoro, B. M. J. Goldthorpe, C. G. Mathew, A. Forbes, D. P. Jewell, and R. J. Playford. 2005. Muramyl dipeptide and toll-like receptor sensitivity in NOD2-associated Crohn's disease. Lancet 3651794-1796. [DOI] [PubMed] [Google Scholar]
- 43.Veazey, R. S., D. W. Horohov, J. L. Krahenbuhl, H. W. Taylor, J. L. Oliver III, and T. G. Snider III. 1995. Comparison of the resistance of C57BL/6 and C3H/He mice to infection with Mycobacterium paratuberculosis. Vet. Microbiol. 4779-87. [DOI] [PubMed] [Google Scholar]
- 44.Veazey, R. S., D. W. Horohov, J. L. Krahenbuhl, H. W. Taylor, J. L. Oliver, and T. G. Snider III. 1996. Differences in the kinetics of T cell accumulations in C3H/HeN (Bcg-resistant) and C57BL/6 (Bcg-susceptible) mice infected with Mycobacterium paratuberculosis. Comp. Immunol. Microbiol. Infect. Dis. 19289-304. [DOI] [PubMed] [Google Scholar]
- 45.Vidal, S. M., D. Malo, K. Vogan, E. Skamene, and P. Gros. 1993. Natural resistance to infection with intracellular parasites: isolation of a candidate for Bcg. Cell 73469-485. [DOI] [PubMed] [Google Scholar]
- 46.Vidal, S. M., E. Pinner, P. Lepage, S. Gauthier, and P. Gros. 1996. Natural resistance to intracellular infections: Nramp1 encodes a membrane phosphoglycoprotein absent in macrophages from susceptible (Nramp1 D169) mouse strains. J. Immunol. 1573559-3568. [PubMed] [Google Scholar]
- 47.Zwilling, B., D. E. Kuhn, L. Wikoff, D. Brown, and W. Lafuse. 1999. Role of iron in Nramp1-mediated inhibition of mycobacterial growth. Infect. Immun. 671386-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]