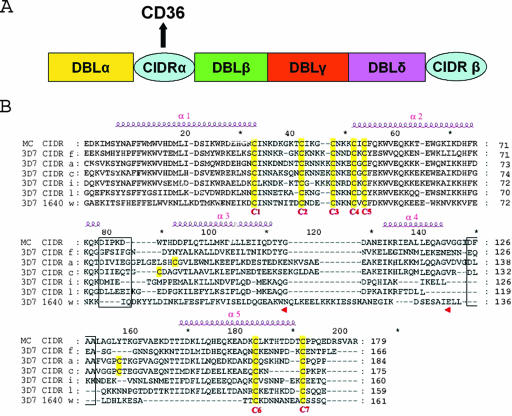
FIG. 1.
(A) Schematic representation of the modular organization in the ectodomain of a PfEMP-1 protein in the 3D7 clone of Plasmodium falciparum. The various modules of PfEMP1 that mediate cytoadhesion (DBLα, -β, -γ, and -δ and CIDRα) are indicated in different colors. The CIDRα domain mediates attachment to the CD36 receptor. (B) Amino acid sequences alignment of CIDR domains from P. falciparum strains 3D7 and MC. Accession numbers for CIDR-f and PFE1640w are given in Materials and Methods. MC CIDR (accession no. AAA89134, residues 576 to 753), CIDR-a (PFA0765c, residues 610 to 793), CIDR-c (PFD0005w, residues 633 to 807, CIDR-i (PFL0005w, residues 589 to 748), and CIDR-l (PFB1055c, residues 583 to 741) are also shown. See reference 31 for a classification of CIDR domains and their CD36 binding function. The seven conserved cysteine residues are numbered C1 to C7 (highlighted in yellow) to follow the convention introduced earlier (4). Predicted α-helices are displayed above the sequences and numbered. Putative loops that connect helices α2-α3 and α4-α5, which were deleted for binding studies (see text), are boxed.