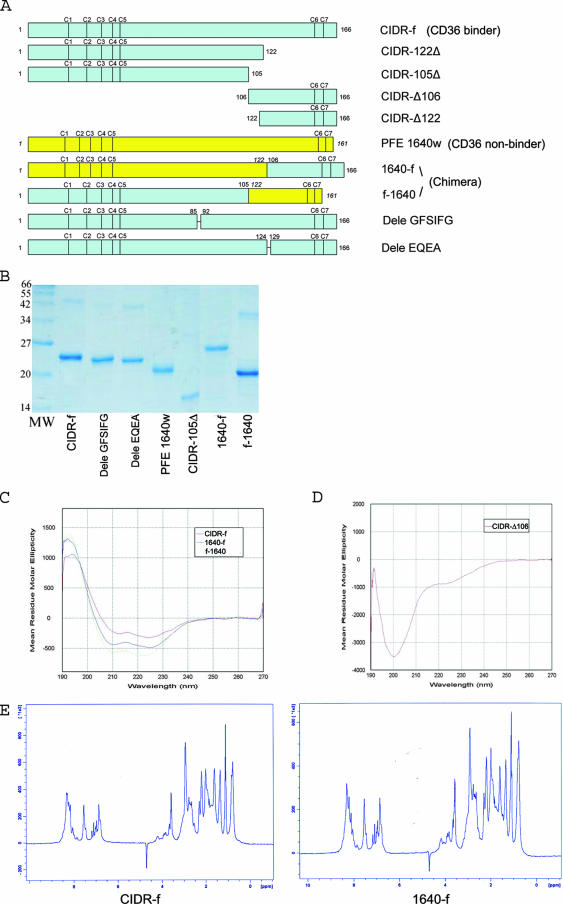
FIG. 2.
(A) Schematic view of the various CIDR constructs and chimeric proteins used for the binding experiments. CIDR-Δ106 and CIDR-Δ122 were expressed in E. coli both as GST fusion proteins and as C-terminal His-tagged fragments (see text). The positions of conserved cysteine residues are indicated. (B) Expression and purification of the various CIDRα mutants and chimeric proteins. All proteins were purified by Ni-nitrilotriacetic acid affinity, ion-exchange, and size-exclusion chromatography and separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. (C) CD spectra of key CIDR constructs and chimeric proteins. The CIDR-f protein and the two chimeric proteins 1640-f and f-1640 all showed similar spectra with one maximum at 190 nm and two minima at 208 nm and 222 nm, indicative of predominantly α-helical proteins. (D) By contrast, the His-tagged CIDR-Δ106 fragment is devoid of secondary structures. (E) 1D 1H NMR spectra of CIDR-f and 1640-f (the CD36 binding active chimera [see text]). The NMR spectra of these two recombinant proteins showed a clear dispersion of resonance lines in the region of amide protons (between 6 and 10 ppm) but downfield-shifted methyl protons (−0.5 to 1.5 ppm) resonances, indicative of globular folded proteins.