Abstract
Recent neuroimaging studies have shown that activity in lateral Heschl’s gyrus covaries specifically with the strength of musical pitch. Pitch strength is important for the perceptual distinctiveness of an acoustic event, but in complex auditory scenes, the distinctiveness of an event also depends on its context. In this magnetoencephalography study, we evaluate how temporal context influences the sustained pitch response (SPR) in lateral Heschl’s gyrus. In 2 sequences of continuously alternating, periodic target intervals and a more irregular baseline interval, the distinctiveness of the target was decreased in 1 of 2 ways—either by increasing the pitch strength of the baseline or by decreasing the pitch strength of the target. The results show that the amplitude of the SPR increases monotonically with the distinctiveness of the target. Moreover, SPR amplitude is greater for the sequence, where the pitch strength of the target is varied, compared with the condition, where the baseline is varied. Two subsequent experiments show that the amplitude of the SPR increases as duty cycle decreases, in a pitch “strength” contrast and in a pitch “value” contrast. These results indicate that the SPR adapts to recent stimulus history, enhancing the response to rare and brief events.
Keywords: auditory event, auditory scene analysis, facilitation, magnetoencephalography, selective adaptation
Introduction
There has recently been a series of neuroimaging studies that demonstrate that there is a pitch-processing center in lateral Heschl’s gyrus (Griffiths and others 1998; Gutschalk and others 2002; Patterson and others 2002; Krumbholz and others 2003; Warren and Griffiths 2003; Gutschalk, Patterson, Scherg, and others 2004; Penagos and others 2004; Rupp and others 2004). There is more activation when a sound has a pitch, and the level of activation varies with pitch strength. Moreover, the fine temporal resolution of magnetoencephalography (MEG) makes it possible to dissociate sustained and transient components of the pitch response (PR). There is a subcomponent of the sustained field (Gutschalk and others 2002) that builds up over the time interval from 60 to 400 ms post stimulus onset (Gutschalk, Patterson, Scherg, and others 2004) and then continues at a fairly stable level while the sound stays on. This component is referred to as the sustained pitch response (SPR). There is also a subcomponent of the transient N1m that peaks 100-150 ms after the onset of a pitch-producing sound. This component is more readily observed when the pitch onset occurs without a concomitant change in sound intensity. It is referred to as the pitch onset response (POR) (Krumbholz and others 2003).
Although these studies have provided insight into how activity in lateral Heschl’s gyrus depends on pitch and pitch strength, little is known about the effect of temporal context on the SPR and POR. The salience of a periodic event in a complex auditory scene depends not only on the pitch strength but also on the distinctiveness of the event, that is, the degree to which it is different from competing auditory events (Bregman 1990; Zatorre and others 2004). (Pitch strength is also referred to as pitch salience. Pitch strength can enhance the general salience of an auditory event, but in the context of multiple events with strong pitch, the salience, or distinctiveness, of a single event may nevertheless be relatively weak. To avoid any confusion between “pitch salience” and salience in general, we will use the term “pitch strength” for the first and “distinctiveness” for the latter throughout this paper).
In this paper, we report studies designed to evaluate how the SPR is influenced by changes in the temporal context surrounding a periodic event, which in these studies is a click train (CT) with a pitch in the region of the human voice, where the interclick interval (ICI) was either constant or jittered to some degree (Pollack 1968). Jittering the ICI reduces the temporal regularity, quantified, for example, by the height of the first peak in the autocorrelation function. This parameter of temporal regularity correlates well with the perceived pitch strength of certain broadband sounds like iterated rippled noise (Patterson and others 1996; Yost 1996); the pitch strength decreases monotonically as the temporal jitter is increased.
Experiment 1 compares the effect of temporal context in 2 forms of sequence, where a periodic target event is alternated with an irregular baseline interval. In one sequence, a periodic, regular CT was alternated with 1 of 4 different baseline sounds with differing degrees of temporal jitter. The stronger the pitch of the baseline epoch, the less distinctive the regular CT is from the baseline epoch. In the second sequence, an irregular CT with little or no pitch was used as a fixed baseline, and it was alternated with 4 target events having varying degrees of pitch strength. In this case, it is the pitch strength of the target that varies. The results showed that the SPR was significantly stronger when the baseline interval was constant and the pitch strength of the target varied from high to low.
The difference in activation might be explained by differential, or selective, adaptation for the target and baseline epochs. This hypothesis was tested in Experiment 2, where a fixed target CT was contrasted with a fixed, aperiodic baseline sound, and the duty cycle was varied, that is, the ratio of target time to baseline time. The results showed that SPR amplitude increases with decreasing duty cycle, that is, with a decrease in the relative duration of the target CT. The strength of these adaptation effects led to a third experiment in which the irregular baseline epoch was replaced by a regular CT in which successive clicks alternated in polarity. When the click rate is low, this alternating-polarity CT produces the same pitch strength as the unipolar CT but the pitch is between 1 and 2 semitones lower. The results showed that adaptation of the SPR can also be produced by temporal contexts with variable pitch value, rather than pitch strength.
Materials and Methods
Listeners
Twelve listeners participated in Experiments 1 and 2, and 6 listeners participated in Experiment 3. The mean age was 28.7 years (6 females, 6 males) in Experiment 1, 29.2 years (6 females, 6 males) in Experiment 2, and 31.3 years (3 females, 3 males) in Experiment 3. All listeners were right handed and had no history of peripheral or central hearing disorder. They provided written informed consent before participating in the experiments.
Stimuli
CTs were presented at 28 dB normal hearing level. Clicks were digitally created at a sampling rate of 48 000 Hz. The length of one click corresponded to one sample, that is, its length was 20.83 μs. The stimuli were presented diotically using ER-3 transducers (Etymotic Research, Inc., Elk Grove Village, IL) with 90-cm plastic tubes and foam earpieces.
Experiment 1: Variation of Pitch Strength
In this experiment, the pitch strength was changed by varying the CT regularity. Periodic CTs, referred to as “target” intervals, were contrasted with more strongly jittered CTs, referred to as “baseline” intervals. Two sequences were compared, in which either the regularity of the baseline (constant target) or the regularity of the target (constant baseline) was changed, to parametrically vary the perceptual distinctiveness of the target event. The mean ICI was 12 ms, and the alternation interval of target and baseline was fixed at 720 ms. The CT regularities were chosen from 100% regular (periodic without jitter), 75% regular (jittered ±1.5 ms), 50% regular (±3 ms), 25% regular (±4.5 ms), or 0% regular (±6 ms). After 25 repetitions, the regularity was switched to the next condition. A total of 200 repetitions of each condition were presented (8 × 25). In the constant target sequence, the target regularity was fixed at 100%, whereas the baseline regularity varied between 75-0%. In the constant baseline sequence, the target regularity was varied between 100% and 25%, whereas the baseline regularity was kept constant at 0% regularity. The regularity contrast in a single condition will be denoted as “% target regularity [% baseline regularity],” for example, 100% [0%] denotes the standard condition where a 100% regular, target CT alternates with a 0% regular baseline. Figure 1 summarizes the stimulus paradigm.
Figure 1.
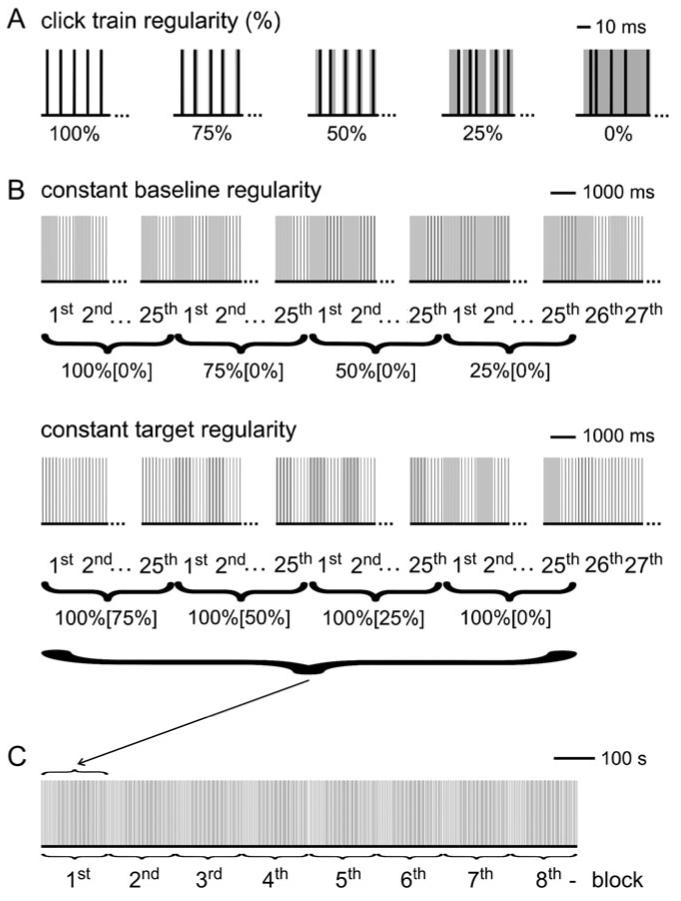
Summary of Experiment 1. (A) Definition of CT regularity in percent; 100% means perfectly regular click positions spaced by the ICI of 12 ms; 75% means clicks were positioned randomly within 25% of the regular click positions, etc. The shaded areas indicate the intervals in which the clicks were jittered. (B) Order of stimulus presentation. The regularity of the target CT was either fixed at 100% or varied between 100% and 25%. The duration of each target and baseline interval was 720 ms, and each condition was repeated 25 times before switching to the next condition, such that 1 block with all 4 conditions lasts 144 s (2 × 720 ms × 25 repetitions × 4 conditions). The contrast of T/B regularity is denoted target% [baseline%]. (C) The 4 different blocks of 25 stimuli were repeated 8 times.
Experiment 2: Variation of the Duty Cycle in a Regularity Contrast
In this experiment, the epoch duration of the regular (100%) and irregular (0% regular) CTs was varied; the mean ICI was again 12 ms. Four conditions were compared: (1:1) duration of regular and irregular CTs was 720 ms; (2:2) duration of regular and irregular CTs was 1440 ms; (1:3) duration of regular CT was 720 ms, whereas duration of irregular CT was 2160 ms; and (3:1) duration of regular CT was 2160 ms, whereas duration of irregular CT was 720 ms. The stimuli were presented in separate sets. The 2:2, 1:3, and 3:1 conditions were presented for 200 repetitions; the 1:1 condition was presented for 400 repetitions to yield equal durations for the stimulus sets (9.6 min). Equal duration of sets rather than an equal number of repetitions was maintained because adaptation of the auditory evoked fields can continue for up to 30 min, and it is strongest in the first few minutes (Näätänen and Picton 1987; Gutschalk, Patterson, Uppenkamp, and others 2004). Presenting the 1:1 condition for 4.8 min (i.e., 200 repetitions), and averaging the less adapted responses only, could have therefore caused an overestimation of the average amplitude for that condition.
Experiment 3: Variation of the Duty Cycle in a Pitch Value Contrast
This paradigm employed 2 different pitch stimuli. One was the regular, unipolar CT with an ICI of 12 ms, as in the experiments above. The second stimulus was a CT with identical parameters except the polarity alternated on successive clicks (Flanagan and Guttman 1960; Patterson 1973). This stimulus is perceived to have a similar pitch strength, but a pitch that is about 10% lower than the pitch elicited by the isochronous CT. The response to these CTs was compared in 2 parts of the experiment. First, CTs with unipolar and alternating polarity were presented in constant alternation with 1 of the 4 relative durations used in Experiment 2 (i.e., 1:1, 2:2, 1:3, and 3:1). Second, regular trains were presented in constant alternation with irregular CTs (0% regularity, polarity matched to regular trains, alternation interval 720 ms). This condition was included to fit the dipoles for the spatial filter and to control for the similarity of the SPR evoked by the unipolar and alternating-polarity CTs. In both parts of Experiment 3, 200 replications of each type were obtained (400 for the 1:1 duty cycle).
Recording and Data Processing
During the recording session, listeners watched a self-selected silent movie to keep their vigilance at a steady level. The MEG was recorded continuously with a 122-channel whole-head gradiometer system (Elekta Neuromag Oy, Helsinki, Finland) and a 1000-Hz sampling rate (330-Hz low-pass filter, no high-pass filter, i.e., direct coupled). The data were averaged off-line with the BESA software (MEGIS Software GmbH, Graefelfing, Germany). Artifact-contaminated epochs were rejected by an automatic gradient criterion and by visual inspection. The length of the epoch was from 400 ms before to 1600 ms (or 3200 ms) after stimulus onset. The baseline was taken from the interval 100 ms before stimulus onset (i.e., the last 100 ms of the baseline epoch) and it was low-pass filtered at 20 Hz (zero-phase shift Butterworth filter, 24 dB/oct).
T1-weighted magnetic resonance imaging (MRI) was obtained from each listener on a 1.5-T MAGNETOM Symphony MRI-scanner (Siemens, Erlangen, Germany). Scans were performed in 176 sagittal slices with a 256 × 256 matrix, yielding an isotropic voxel size of 1 mm3. Dipole locations were coregistered on individual MRIs, and the positions were then transformed to the coordinate system of Talairach (Talairach and Tournoux 1988) for further evaluation using Brainvoyager (Brain Innovation B.V., Maastricht, The Netherlands).
Source Analysis
Spatiotemporal dipole source analysis (Scherg 1990) was used to create a spatial filter for the analysis of the PR, consisting of one dipole in each auditory cortex. For comparability across conditions, this part of the spatial filter was fixed. We defined the PR to be the N1m plus the sustained field elicited by pitch after the baseline activity of a nonpitch sound is subtracted (Gutschalk and others 2002; Krumbholz and others 2003; Gutschalk, Patterson, Scherg, and others 2004). The N1m of the PR will be referred to as the POR (Krumbholz and others 2003). The sustained field of the PR will be referred to as the SPR (Gutschalk, Patterson, Scherg, and others 2004). The dipoles were fitted to the SPR of the 100% [0%] contrast because 1) it typically provided the best signal-to-noise ratio and 2) it was available for all 3 experiments. In all cases, the SPR dipoles were fitted in the interval from 400 to 720 ms post stimulus onset. In Experiment 1, the dipoles were fitted to a grand average that comprised the 100% [0%] contrast of the 2 sets presented here, as well as a third set published previously (Gutschalk, Patterson, Scherg, and others 2004) to yield a total of 600 repetitions for each subject. In Experiment 2, the 1:1 condition with 400 repetitions was used for source analysis, and in Experiment 3, an average of the unipolar and alternating-polarity trains in the regular:irregular contrast was used. An individual example, taken from Experiment 1, showing the sensor waveforms used for dipole localization and the corresponding dipole positions and source waveforms is illustrated in Figure 2.
Figure 2.
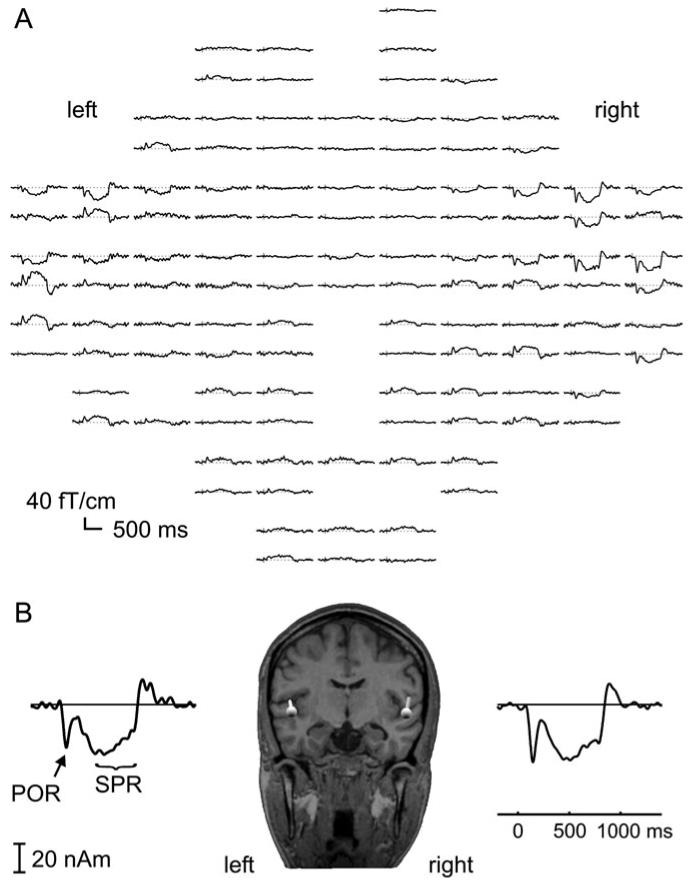
Exemplary data set used for source analysis in a single subject of Experiment 1 (600 replications of a 100% [0%] regularity contrast). (A) Sensor waveforms are shown in top view. The gradiometer system used comprises 122 planar gradiometers (figure-of-eight coils) in 61 locations, evenly distributed around the head. Waveforms of the 2 orthogonal gradiometers at each position are plotted above each other. Five channels were not used in this recording because of high sensor noise. The time window is from 200 ms before target onset to 1400 ms after target onset. The data have been low-pass filtered at 20 Hz, and a baseline is set 100 ms before target onset. (B) Shows the dipole positions, fitted to the SPR in the interval from 400 to 720 ms after target onset, coregistered to the subjects anatomical MRI. The dipole source waveforms, corresponding to the data set shown in (A), are plotted for the left and right dipole, respectively.
To model any remaining drift and other low-frequency artifacts, a principal component analysis (PCA) was computed over the interval 1340-1440 ms (or 2780-2880 ms) post stimulus onset, that is, the repetition of the interval where the baseline was set. The PCA component accounting for most variance in this interval was then added to the spatial filter for the respective condition. This part of the spatial filter had to be individually adjusted for each experimental condition. In the case of eye movement artifacts, blinks were averaged and another PCA component explaining the blinks was included in the spatial filter (Berg and Scherg 1994). Similarly, in some listeners high-amplitude alpha rhythm was modeled with 5-10 PCA components derived from the raw data. The spatial filter to extract source waveforms was then obtained by combination of the dipole’s lead field vectors with the PCA topographies modeling the artifacts.
The source amplitude of sustained fields was measured in the interval 400-720 ms after target CT onset. The amplitude of the transient fields was taken to be at the peak amplitude and, in 2 conditions, is reported as the peak-to-peak amplitude. Separate analyses of variance were calculated for the latencies, amplitudes, and dipole locations with the SAS package (SAS Institute Inc., Cary, NC). When appropriate, the significance level was adjusted with the Greenhouse-Geisser correction for the degrees of freedom; significance levels were not corrected for multiple comparisons.
Dipole models for the transient peaks were fitted to compare their source locations, but they were not used for the spatial filter. Given the close proximity of the SPR and POR sources, separate dipoles provide little extra information (Gutschalk, Patterson, Scherg, and others 2004); moreover, they often cause strong enhancement of the noise level in the source waveforms. For dipole analysis of the transient waves, the data were high-pass filtered at 3 Hz (zero-phase shift Butterworth filter, 12dB/oct) to suppress the overlapping SPR. The dipoles were then fitted in an interval 30 ms in duration around the individual peak’s latency.
Results
Experiment 1: Variation of Pitch Strength
Grand average source waveforms for all pitch contrasts are shown in Figure 3. The waves on the left were recorded in the constant target (variable baseline) sequence and the waves on the right in the variable target (constant baseline) sequence. The SPR dipoles used in the spatial filter were located in lateral Heschl’s gyrus (cf., Gutschalk, Patterson, Scherg, and others 2004); the mean Talairach coordinates are listed in Table 1. The source waveforms show that the activity was very similar in left and right hemispheres and so the results will be described in terms of the combined activation.
Figure 3.
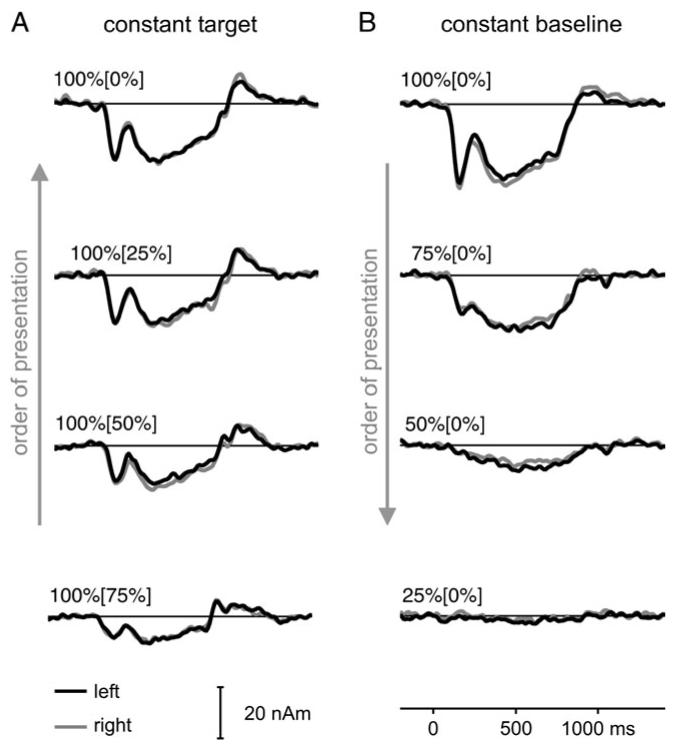
Grand average source waves for Experiment 1. Activity from the left (black) and right auditory cortex (gray) is shown in the same panel. The inserts show the regularity values for the target and, in brackets, the baseline. The data represent an average over 12 subjects, where each trace is based on 200 replications per subject. (A) Constant target regularity with variable baseline regularity. (B) Constant baseline regularity with variable target regularity. Arrows indicate the order of stimulus presentation (cf., Fig. 1).
Table 1.
Dipole locations in the space of Talairach and Tournoux (1988)
| Condition | auditory evoked field-component | Talairach coordinates (x, y, z; mean ± standard deviation) |
|
|---|---|---|---|
| Left auditory cortex | Right auditory cortex | ||
| Experiment 1 | |||
| 100% [0%]a | SPR | −48 ± 5, −15 ± 2, 5 ± 6 | 47 ± 5, −10 ± 6, 6 ± 5 |
| POR | −47 ± 6, −20 ± 3, 4 ± 7 | 47 ± 4, −11 ± 7, 8 ± 6 | |
| Experiment 2 | |||
| 1:1 | SPR | −50 ± 9, −13 ± 5, 7 ± 11 | 49 ± 7, −8 ± 7, 10 ± 10 |
| POR | −48 ± 7, −19 ± 5, 7 ± 11 | 50 ± 6, −12 ± 7, 10 ± 4 | |
| 1:3 | SPR | −49 ± 9, −17 ± 9, 7 ± 9 | 46 ± 10, −11 ± 9, 7 ± 11 |
| POR | −51 ± 4, −17 ± 4, 11 ± 8 | 52 ± 5, −13 ± 6, 8 ± 6 | |
| 3:1b | N1m-off | −50 ± 11, −19 ± 9, 10 ± 11 | 51 ± 6, −18 ± 7, 10 ± 10 |
| P2m-off | −53 ± 9, −19 ± 9, 9 ± 12 | 53 ± 11, −11 ± 9, 13 ± 6 | |
| Experiment 3 | |||
| 1:1 | SPR | −46 ± 5, −13 ± 4, 9 ± 7 | 51 ± 7, −11 ± 8, 8 ± 5 |
| POR | −50 ± 3, −19 ± 4, 5 ± 8 | 51 ± 5, −12 ± 5, 10 ± 8 | |
| 1:3 + 3:1 | SPRc | −47 ± 5, −16 ± 4, 13 ± 4 | 49 ± 5, −9 ± 7, 12 ± 11 |
| Pitch change N1m | −55 ± 6, −19 ± 7, 6 ± 12 | 55 ± 5, −12 ± 7, 11 ± 13 | |
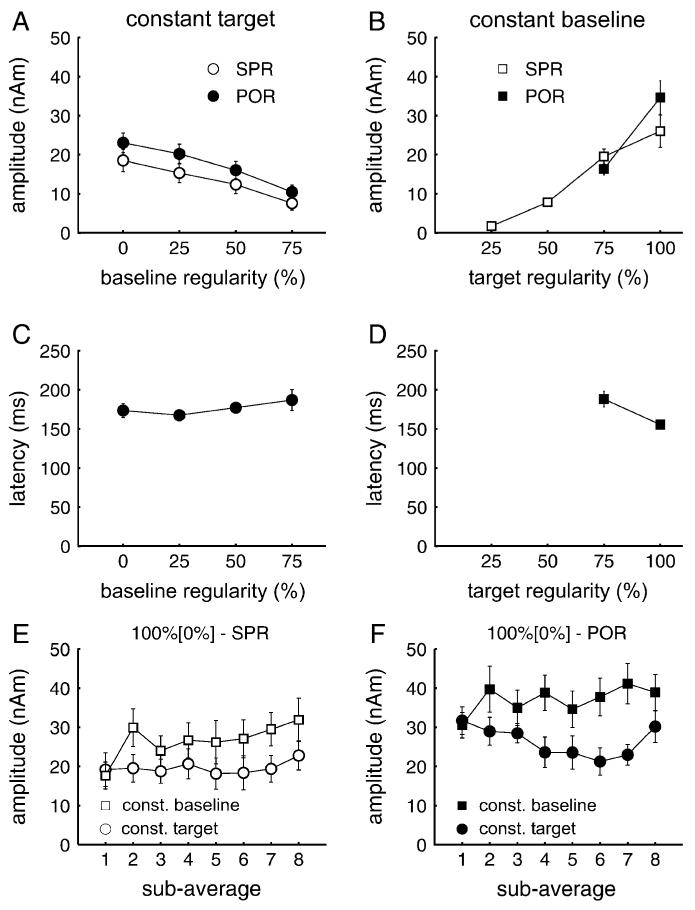
There was a monotonic relationship between target distinctiveness and the amplitude of the PR within each sequence. This was expected when the SPR varied with the regularity of the baseline as well as the target interval. In the constant target condition, the amplitude of the PR decreased with increasing regularity of the baseline interval, that is, the PR decreased with decreasing perceptual contrast between baseline and target (F3,33 = 20.48, P < 0.0001). There was no significant difference in the behavior of the SPR and the POR (PR component × regularity: F3,33 = 1.01, not significant [NS]; cf., Fig. 4A). The mean latency of the POR varied between 167.5 and 187.0 ms, but there was no significant effect of baseline regularity on POR latency (F3,33 = 1.87, NS; cf., Fig. 4C).
Figure 4.
(A and B) Amplitudes of the SPR (white) and POR (black). The POR was measured at its peak in the individual source waveforms of each subject (interval 80-250 ms). The SPR was measured as the average amplitude in the interval from 400 to 720 ms after target onset. (C and D) Peak latency of the POR (A, C constant baseline; B, D constant target). Mean amplitude of (E) the SPR and (F) the POR, for each of the 8 blocks of the 100% [0%] condition. Circles represent the constant target condition, squares the constant baseline condition. Error bars indicate the standard error of the mean.
In the constant baseline condition, the PR decreased monotonically with decreasing target regularity (SPR: F3,33 = 29.49, P < 0.0001). With reference to bootstrap-based student’s t-intervals (1000 resamples), there was no significant level of PR (POR and SPR) in the 25% [0%] condition. There was also no significant POR peak in the 50% [0%] condition. That is, the SPR decreased less than the POR as target regularity decreased (Fig. 4B). The latency of the POR was longer (188.3 ± 10.1 ms) for targets with 75% regularity than 100% regularity (155.4 ± 5.0 ms; cf., Fig. 4D).
Comparison of the POR and SPR across sequence type revealed larger amplitudes for the constant baseline sequence. The SPR and the POR of the 100% [0%] contrast were significantly larger in the constant baseline sequence (F1,11 = 22.96, P < 0.001), and the SPR amplitude for the 75% [0%] contrast was still about the same size as for the 100% [0%] contrast in the constant target sequence (19.5 ± 1.0 vs. 18.5 ± 2.8 nAm). The amplitudes of the POR and the SPR were measured separately for each of the 8 blocks in the design (cf., Fig. 1) to evaluate how this difference evolved and whether there was longer term adaptation. Panel E in Figure 4 shows the blockwise SPR amplitudes and panel F the blockwise POR amplitudes for the 100% [0%] contrast. During the first block, the amplitudes of the SPR and POR are of similar size for both sequences. Thereafter, the amplitudes are significantly larger in the constant baseline sequence (difference over all repetitions: F1,11 = 23.93, P < 0.001). The amplitude increment of the PR in the constant baseline sequence was significant (F7,77 = 3.58, P < 0.05); there was no significant effect of block for the constant target sequence (F7,77 = 0.99, NS). There was also no significant effect of block beyond the first block in the constant baseline sequence (constant baseline block 2-8: F6,66 = 1.35, NS).
The change in the amplitude of the PR from the first to the second block in the constant baseline condition appears to be closely related to the order of the stimulus sequence; as the constant baseline sequence started with the 100% [0%] contrast (cf., Fig. 1), sequence effects for this contrast could only be expected from the second block on. In contrast, the 100% [0%] contrast was the last element in the constant target sequence and thus always followed the same local sequence. Although the 100% [0%] contrast occurred with the same frequency in both sequences, the proportion in which the single elements occurred was reversed in the 2 sequences: In the constant baseline sequence, the 0% regularity element accounted for half of the total sequence time and the 100% regularity element for only one-eighth. In the constant target sequence, the 0% regularity element accounted for one-eighth and the 100% regularity element for half of the sequence time. Thus, the amplitude evoked by the contrast was stronger when the target was less frequent and the baseline more frequent, or summarized in one measure, when the ratio of target time to baseline time was decreased.
Experiment 2: Variation of the Duty Cycle in a Regularity Contrast
Experiment 2 was designed to test whether the SPR is directly related to the ratio of target time to baseline time within a sequence. It employed a simpler sequence, comprising only the 100% [0%] contrast, where the duty cycle (i.e., the fraction of target time relative to the duration of one cycle) was varied as well as the duration of the cycle itself. The SPR was expected to increase when the duty cycle of the target was decreased, that is, when the target duration was decreased and the baseline duration increased by the same amount. Conversely, the SPR was expected to decrease when the target duty cycle was increased. No amplitude change was expected when both intervals shared the same duty cycle, but the total duration of the cycle was varied. The latter comparison was included to control for the possibility that the SPR might depend on the interstimulus interval (ISI) or the stimulus onset asynchrony (SOA), as suggested by earlier reports on the sustained potential recorded with pure tones (Picton and others 1978b) (we define the ISI as the interval between the end and the beginning of a succeeding target and the SOA as the interval between their onsets, i.e., it is independent of target duration).
We compared 4 different target-to-baseline (T/B) ratios; the T/B ratio was 1:1 (720:720 ms), 2:2 (1440:1440 ms), 1:3 (720:2160 ms), or 3:1 (2160:720 ms). Panel A in Figure 5 shows the grand average source waves derived with the SPR dipoles. The stimulation is schematically illustrated below the waves. Relative to the 1:1 activity, the SPR was a little larger for a T/B of 1:3 and much smaller for a T/B of 3:1. The effect of duty cycle on the SPR was significant (F3,33 = 22.67, P < 0.0001). There was no significant amplitude difference when ISI was doubled, that is, between T/Bs of 1:1 and 2:2 (measured in the interval 400-720 ms; F1,11 = 1.80, NS). This observation confirms the hypothesis that the SPR depends on the duty cycle and not on the ISI.
Figure 5.
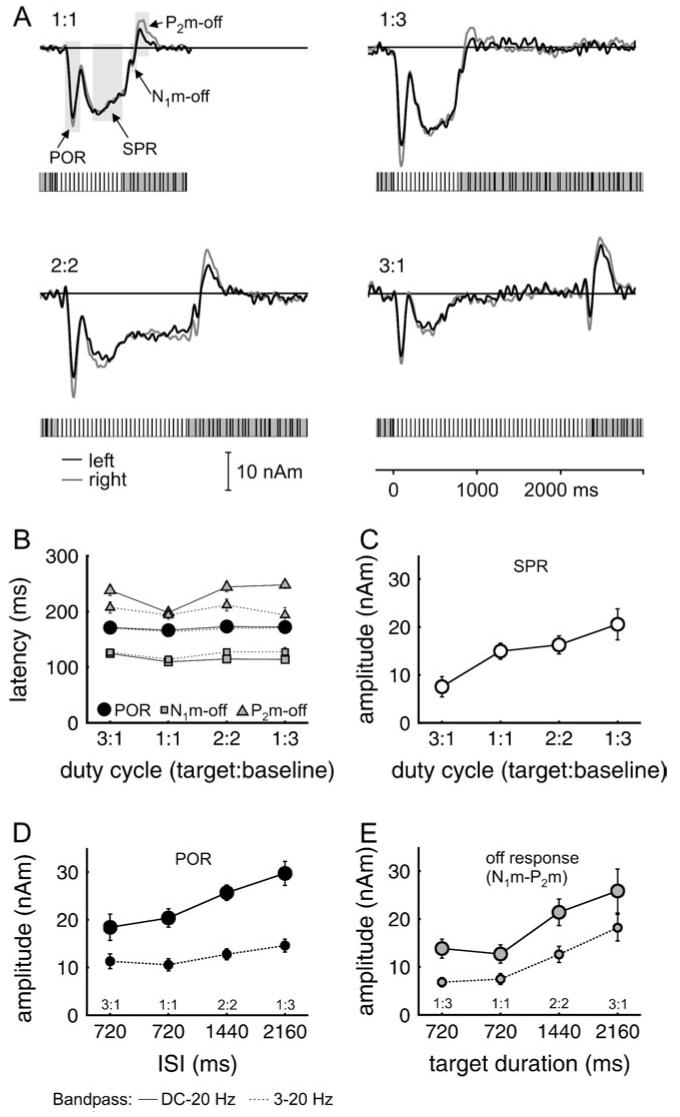
(A) Grand average source waves for Experiment 2. The data represent an average over 12 subjects, where each trace is based on 200 (1:1, 400) replications per subject. Activity from the left (black) and right auditory cortex (gray) is shown in the same panel. The stimuli are shown schematically below the source waves. The duty cycle is indicated as target-to-baseline ratio (1:1, 1:3, 2:2, and 3:1). Shaded areas in the left upper panel indicate the measurement intervals for the different waves. (B) Mean latencies for the POR (black circle), the N1m-off (gray squares), and the P2m-off (gray triangles). Symbols connected by solid lines represent values measured without high-pass filter (like the source waves in (A) and those connected by dashed lines were measured with a 3-Hz high-pass filter (Butterworth, zero-phase shift). The high-pass filter eliminates the SPR and simplifies the measurement of the peak maxima. (C) Mean amplitudes of the SPR from 400 to 720 ms (white circles). (D) Mean amplitudes of the POR in the measurement interval from 80 to 250 ms (black circles). (E) Mean peak-to-peak amplitude of the offset N1m-P2m (gray circles). N1m measurement interval 100-160 ms (90-180 ms), P2m measurement interval 150-300 ms (130-300 ms). Error bars indicate the standard error of the mean.
The amplitude of the POR, on the other hand, did vary with the duration of the baseline interval (i.e., the ISI; main effect over conditions: F3,33 = 17.14, P < 0.0001). The amplitude increased monotonically with ISI increasing from 720 to 2160 ms. There was no significant difference between conditions with T/Bs of 1:1 and 3:1, which shared the same ISI (F1,11 = 1.40, NS). There was also a transient response at pitch offset (i.e., the transition from regular to irregular), consisting of an N1m and a P2m. A small P1m was also observed for a T/B of 3:1. To distinguish these peaks from the onset waves, we will refer to them as P1m-off, N1m-off, and P2m-off. The latency of these waves is presented in Figure 5B. Similar to the dependence of the POR on ISI, the peak-to-peak amplitude (measuring the peak-to-peak amplitude reduces distortions of the baseline caused by interactions of N1m, P2m, and the falling slope of the SPR) of the off response (N1m-P2m) increased monotonically with the duration of the regular target interval (main effect over conditions: F3,33 = 15.29, P < 0.001). There was no significant difference between conditions with T/Bs of 1:1 and 1:3, which share the same duration of target interval (F1,11 = 4.19, NS).
To estimate the location of the source of the off response, dipoles were fitted for the N1m-off and the P2m-off using the 3:1 condition. This was possible in 10 out of 12 listeners. Mean Talairach coordinates for the off response are provided in Table 1, together with those for the SPR and POR. There was no significant difference between the locations of the N1m-off and P2m-off dipoles (F2,18 = 1.52, NS). The off-response dipoles were also not significantly different from those for the POR in the same listeners (N1m-off vs. POR: F2,18 = 1.06, NS; P2m-off vs. POR: F2,18 = 0.71, NS). The average location of the SPR and POR was in lateral Heschl’s gyrus with respect to normative data (Leonard and others 1998), and it was consistent with the locations found in Experiment 1.
In half of the listeners, not only the amplitude of the SPR was reduced for the condition where T/B was 3:1 but also its polarity was inverted from surface negative to surface positive in one or both hemispheres. This might be explained either by a change in the equilibrium of negative and positive currents within one cortical field or by an enhancement of negative source activity during the irregular interval. When the baseline in the 3:1 condition is set 100 ms prior to the transition from regular to irregular, a low-magnitude, negative sustained field is observed during the shorter, irregular interval in these listeners. The signal-to-noise ratio of this negative field was insufficient for dipole source analysis, however. Dipoles fitted to the SPR for the inverse condition, with T/B equals 1:3, were located slightly but not significantly posterior to those fitted for T/B equals 1:1 (F2,22 = 0.52, NS; cf., Table 1).
Experiment 3: Variation of the Duty Cycle in a Pitch Contrast
In Experiments 1 and 2, the distinctiveness of the target CTs was determined by their stronger pitch compared with the baseline interval. In Experiment 3, we used 4 sequences similar to the ones in Experiment 2, but in this case, they were segregated by a contrast in pitch value instead of pitch strength. The purpose was to test the hypothesis that the differential adaptation of the SPR is a general property of the source in lateral Heschl’s gyrus, when presented with a stimulus in a distinctive temporal context. The click rate, intensity, and regularity of the target and baseline were matched, while maintaining a perceptual distinction by alternating the polarity of successive clicks in the train. The pitch of this sound is usually perceived to be about 10% lower (or higher) than that of the unipolar CT, depending on the instructions and the listener (Flanagan and Guttman 1960; Patterson 1973).
Figure 6A shows the PR for both the unipolar and alternating-polarity CTs. In this control condition, the trains were alternated with an irregular baseline. A grand average of the unipolar and alternating-polarity data was then used to fit the dipoles to the SPR. Except for subtle differences, the shape and the amplitude of the PR source waves were essentially the same for the unipolar and alternating-polarity CTs. However, the evoked response changed markedly when there was no irregular interval and the sequence of stimulation alternated between 2 regular CTs that only differed in pitch. The source waves for the 4 conditions are shown in Figure 6B. As expected, there was no consistent shift in SPR when the 2 CTs have the same duration (either 720 or 1440 ms) and thus can be expected to produce the same amount of SPR. The transitions only produce the late negative wave, which is also observed riding on the SPR. However, when the duty cycle is 1:3 or 3:1, there is a sustained, negative shift during the “shorter” CT, which is the unipolar train in 1:3 and the alternating train in 3:1. The mean size of this sustained shift was 8-10 nAm (Fig. 6D), which is similar in size to the adaptation effect observed in Experiments 1 and 2.
Figure 6.
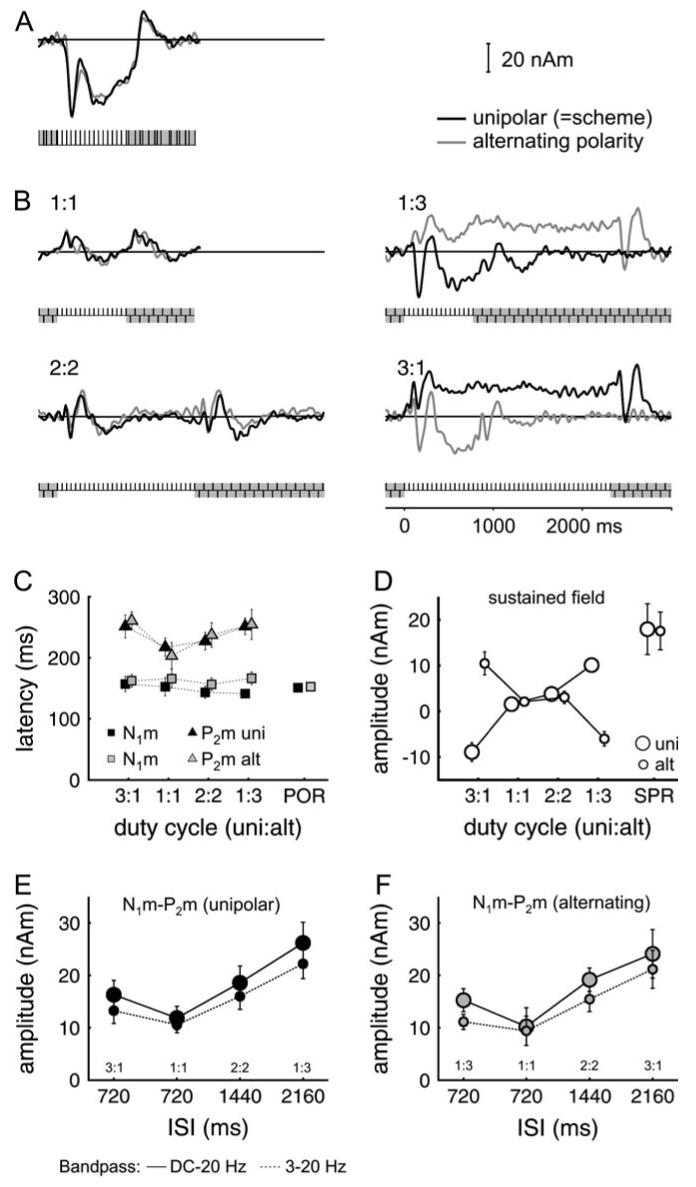
Grand average source waves, latencies, and amplitudes for Experiment 3. The data represent an average over 6 subjects, where each trace is based on 200 (1:1, 400) replications per subject. The activity is averaged over right and left hemispheres. (A) Control condition: periodic unipolar (black) and alternating-polarity (gray) CTs were alternated with irregular CTs. (B) Continuous alternation of unipolar and alternating-polarity CTs (not interspersed with irregular CTs). The baseline was set 100 ms before the onset of the unipolar CT (black curves), corresponding to the schematic stimulus (t = 0 ms is aligned to the onset of the unipolar CT). For comparison, the baseline was also set before the onset of the alternating-polarity CT (gray curves, no corresponding scheme), and the onset was aligned to the same position. The duration of unipolar and alternating-polarity CTs was 720:720 ms (1:1), 1440:1440 ms (2:2), 720:2160 ms (1:3), or 2160:720 ms (3:1). (C) Mean latencies of the N1m and P2m at the transition from alternating polarity to unipolar (black) and at the transition from unipolar to alternating polarity (gray). N1m measurement interval 100-250 ms, P2m measurement interval 150-350 ms. (D) Amplitudes of the sustained field shift (average in the interval 400-720 ms), (E) Peak-to-peak amplitudes (N1m-P2m) for the transient response evoked at the transition from alternating polarity to unipolar, and (F) at the transition from unipolar to alternating polarity. Small circles indicate the unipolar/alternating transition and large circles the alternating/unipolar transition. Error bars indicate the standard error of the mean. Symbols connected by solid lines represent values measured without high-pass filtering (like the source waves in A); those connected by dashed lines were measured with a 3-Hz high-pass filter (Butterworth, zero-phase shift).
The transient response evoked by the pitch change was triphasic, with the usual form, namely, P1m, N1m, and P2m. The latencies of the positive peaks around 80-90 and 200-280 ms were close to the latencies of the waves observed at pitch offset in Experiment 2. In contrast, the latency of the N1m produced by a pitch change was about 40 ms longer than the N1m-off and only slightly shorter than the POR in the control condition (Fig. 6C). However, the amplitude of this N1m was a factor of 2-4 smaller than that of the POR for the same ISI.
The peak-to-peak amplitude of the transition from N1m to P2m, following a pitch change, adapted to the ISI, as did the POR and the off response in Experiment 2. In contrast to Experiment 2, however, the transition from N1m to P2m was not the same size in the 1:1 and 1:3 conditions when the duration of the preceding interval is the same. In this case, the longer SOA in the 1:3 and 3:1 conditions appeared to have some influence on the amplitude of the transient response as well.
Using an average of the data from the 1:3 unipolar and alternating conditions, it was possible to fit a bilateral dipole model for the pitch change N1m in all listeners and for the sustained shift in 5 of 6 listeners. The locations of the dipoles fitted for the sustained shift were close to those for the SPR in the regular-irregular contrast. The locations of the dipoles fitted to the pitch change N1m were slightly lateral (about 0.5 cm) to the dipoles fitted to the POR. The mean Talairach coordinates are listed in Table 1. When these average dipole locations are compared with the mean sulcal borders of Leonard and others (1998), their location is found to be in lateral Heschl’s gyrus.
Discussion
Our data demonstrate that the amplitude of the SPR is affected by the duty cycle of the target source within the temporal context of a sequence. The SPR is stronger when the duty cycle of the periodic event is smaller and when the duty cycle of the baseline event is larger. The SPR recorded with a continuous stimulation paradigm is a difference response (Gutschalk, Patterson, Scherg, and others 2004). Lateral Heschl’s gyrus and surrounding areas are activated by broadband noise, already (Harms and Melcher 2002; Patterson and others 2002), and the specificity for pitch processing causes a “response enhancement” over the baseline level evoked by nonpitch sounds. The absolute response level, relative to a silent baseline, cannot be determined in a continuous paradigm. As a result, 3 principles could account for the enhancement of the SPR: an increment of the response to the less frequent sound (facilitation), a decrement for the more frequent sound in the sequence (adaptation, habituation), or a combination of both.
Selective Adaptation and Facilitation for Pitch
The response enhancement in Experiment 1, and the reversal of polarity of the SPR observed for a number of listeners in Experiment 2, could be explained without facilitation, if we assume that the SPR and the baseline activity adapt differently, depending on the duty cycle. Selective adaptation for the auditory event presented longer or more frequently could then explain the results of our experiments without further assumptions.
Ulanovsky and others (2003) recently showed selective adaptation in single neurons of A1 in halothane-anesthetized cats for pure tones varied in frequency or amplitude. In their study, the response strength was inversely related to the probability of a specific stimulus within a sequence of 2 sounds that were randomly alternated at a fixed probability (so called oddball paradigm). Most prominently, selective adaptation was observed for the sustained part of the neural response. The relative probability in an oddball paradigm and the duty cycle within a continuous sequence are related in so far that they express the temporal relation of the occurrence of 2 sounds in a sequence. However, in the oddball paradigm, the 2 stimuli do not alternate; rather, the more frequent one is presented repeatedly, interrupted by a silent baseline. Therefore, the duty cycle is generally lower than in the continuous stimulation paradigm used here. Selective adaptation may represent one organizing principle found in a subset of cortical, as well as subcortical networks (Malone and Semple 2001). In the visual cortex, for example, selective adaptation has been described for structural patterns (Movshon and Lennie 1979), color contrast (Engel and Furmanski 2001), orientation (Boynton and Finney 2003), and motion (Huk and others 2001). Eytan and others (2003) showed that selective adaptation can be observed in some cultured networks of cortical neurons, and they reported time constants in the range of a few minutes for selective adaptation in these networks. In the auditory cortex of anesthetized cats, selective adaptation can occur within a few seconds, but it may also take several minutes depending on the temporal stimulus context (Ulanovsky and others 2004).
The data from the current paper place only broad constraints on the time constants involved: in Experiment 1, a steady state was reached after one block of 108 s. On the other hand, it appears that the process lasts longer than the 36 s it took for the 25 cycles of one condition in Experiment 1. The SPR for the 75% [0%] contrast in the constant baseline condition was at least as strong as the 100% [0%] contrast in the constant target condition, which would not be expected if the response adapted within few seconds. Moreover, the equivalence of the SPR for the 720:720-ms and the 1440:1440-ms conditions in Experiments 1 and 2 might suggest that the process is at least an order of magnitude longer than the 3-s base interval.
However, it might well be that the effect of temporal context on the SPR is not just adaptation in the physiological sense (i.e., stimulus response decay over time): Barlett and Wang (2005) have recently presented evidence of suppression, as well as facilitation between amplitude-modulated sounds in single auditory cortex neurons of awake monkeys. They compared long-duration sound pairs that differed in either carrier frequency or modulation frequency and found that suppression was more frequently observed for similar sounds, whereas facilitation was observed for dissimilar sounds. Both of these observations match with the SPR data. Moreover, the response modulation in their study was present for more than 1 s, and it involved sustained firing neurons (Barlett and Wang 2005). Although these findings are generally consistent with the concept of selective adaptation for the SPR, they also suggest that facilitation may contribute to the temporal context effect.
Specificity for Pitch in Auditory Cortex
Our data provide further evidence for the presence of a pitch center in lateral Heschl’s gyrus (Griffiths and others 1998; Gutschalk and others 2002; Gutschalk, Patterson, Scherg and others 2004; Patterson and others 2002; Krumbholz and others 2003; Penagos and others 2004). They replicate the location of a source that is responsive to a change in pitch strength in lateral Heschl’s gyrus. Moreover, the location of the adaptation of the SPR and the N1m to a pitch change (Experiment 3) is close-by. Adaptation for a certain feature has frequently been thought of as indicating specificity, for example, in studies of higher order visual fields (Buckner and others 1998; Grill-Spector and others 1999; Huk and others 2001). Warren and Griffiths (2003) adopted this concept for an auditory functional magnetic resonance imaging (fMRI) study and showed that variation of pitch enhances the activity in lateral Heschl’s gyrus, overlapping and somewhat lateral to the activation for pitch versus nonpitch contrasts (Griffiths and others 1998; Patterson and others 2002). Our data suggest that the static and variable contrasts in fMRI represent a mixture of sustained and transient neural activity, which are, respectively, the SPR and the POR. Compared with probabilistic maps of human auditory cortex histology (Rademacher and others 2001), the dipole locations found for the SPR are at the lateral end of the auditory konio field. Because the extent of the related neural generator cannot be estimated with dipole source analysis, the histological fields that could be involved in the PR (and its contextual modulation) are the (hypothetical) human analogue of monkey area R (hR) (Morel and others 1993; Morosan and others 2001; Formisano and others 2003), the neighboring belt fields (Braak 1978, Galaburda and Sanides 1980), as well as the more medial A1 (Braak 1978; Morosan and others 2001). Bendor and Wang (2005) recently provided evidence that there are sustained firing neurons in awake monkeys that respond to the missing fundamental, a low pitch that is heard in the absence of energy at that frequency. It is worth noting that they also presented CTs (Bendor and Wang 2005) and that, like the SPR in the present study, the activity of pitch-sensitive neurons decreased with increasing temporal jitter within CTs. Pitch neurons were only found in a focal area close to the low-frequency boarder of A1 and R and the lateral belt fields (Bendor and Wang 2005). The location of this focal area is in agreement with previous neuroimaging data (Griffiths and others 1998; Gutschalk and others 2002; Patterson and others 2002), as well as the dipole locations in the present study. Together these studies indicate that there is probably a relationship between the 2 areas. However, we do not yet know whether the generator of the SPR and the sites involved in its contextual modulation are as focal as the pitch area in monkey auditory cortex or whether they also include additional activity from neighboring sites.
Transient and Sustained Fields
In Experiment 2, the POR and the N1m-P2m at pitch offset varied with the ISI. This parallels the observations for the N1m with silent ISI (Imada and others 1997) and the relation of the off response to the stimulus duration (Hillyard and Picton 1978). Moreover, previous studies have shown that the ISI dependency is a general feature of N1m subcomponents (Mäkelä and others 1988; Lü and others 1992). The N1m-P2m at pitch change was also related to the ISI, but some increase was observed with increasing SOA as well. Although the N1m at pitch change has a similar latency as the POR, its amplitude is much smaller than the POR for the 100% [0%] contrast at the same ISI. This is probably related to the relatively weak perceptual contrast; the 2 CTs were mostly perceived as 1 stream of 2 tones alternating up and down by 1 semitone, corresponding to pitches of 83 and 78 Hz. For larger tone intervals, the size of the pitch change N1m might increase to levels like those observed for the pitch strength POR (cf., Figs 3 and 4). It is interesting to note, however, that the amplitude difference between ISI conditions was similar for Experiments 2 and 3, both for the transient response and the sustained shift.
Picton and others (1978b) showed that the sustained potential evoked by pure tones increases with SOA when the tone duration is held constant. When they kept the SOA fixed and increased the duration of the tone, the sustained potential decreased. These data might imply that the sustained response is related to the ISI, similar to the N1m. The data from Experiments 2 and 3 show that the interaction of SOA and duration can be explained by one variable—the duty cycle. Another difference between the adaptation observed for the sustained field and the N1m is the time course. Although a precise estimate is not yet available, it appears that the modulation of the SPR, and possibly more generally the sustained field, takes tens of seconds (cf., previous paragraph). In contrast, the adaptation of the N1 to the ISI only requires a few repetitions (Ritter and others 1968; Budd and others 1998). Although there is sometimes additional, gradual adaptation over tens of minutes (Näätänen and Picton 1987; Gutschalk, Patterson, Uppenkamp, and others 2004), this was observed for neither the transient nor the sustained response in the present study.
Ulanovsky and others (2003) suggested that selective adaptation at the single-neuron level might be closely related to the mismatch negativity (MMN). There has been an ongoing discussion whether the MMN is determined by the interdeviant interval or the probability of the deviant within the sequence (cf., Picton and others 2000). If selective adaptation of the N1 (Butler 1968) causes the MMN (May and others 1999; Jääskeläinen and others 2004), then the interdeviant interval should be its determinant. Alternatively, selective adaptation of the sustained field could explain an MMN determined by the probability of the deviant. Possibly, both the N1m and the sustained field need to be considered to explain the MMN with the concept of selective adaptation because a small sustained response is elicited even by short, transient sounds (Picton and others 1978a). In an MMN study that used stimuli long enough to record a strong sustained response, Scherg and others (1989) presented a source decomposition that supports this hypothesis: Deviant stimuli evoked a larger N1 that was separated from a second MMN source associated with a longer response latency, and this source also picked up a clear sustained shift.
It appears that the modulation of the N1m and the sustained field reflects 2 general mechanisms for the representation of temporal context in auditory cortex: the transient N1m mechanism represents temporal intervals between similar events. The sustained field mechanism adapts to the relative probability of a sound pattern, possibly enhancing the representation of variable events over competing, more constant, events. These mechanisms might compare with the representation of edges (Fishbach and others 2001) and continuous texture within figure and ground in vision.
Acknowledgments
The research was supported by the Deutsche Forschungsgemeinschaft (GU-593/2-1, RU-652/1-3) and the UK Medical Research Council (G9900369). Conflict of Interest: None declared.
References
- Barlett EL, Wang X. Long-lasting modulation by stimulus context in primate auditory cortex. J Neurophysiol. 2005;94:83–104. doi: 10.1152/jn.01124.2004. [DOI] [PubMed] [Google Scholar]
- Bendor D, Wang X. The neuronal representation of pitch in primate auditory cortex. Nature. 2005;436:1161–1165. doi: 10.1038/nature03867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg P, Scherg M. A multiple source approach to the correction of eye artifacts. Electroencephalogr Clin Neurophysiol. 1994;90:229–241. doi: 10.1016/0013-4694(94)90094-9. [DOI] [PubMed] [Google Scholar]
- Boynton GM, Finney E. Orientation-specific adaptation in human visual cortex. J Neurosci. 2003;23:8781–8787. doi: 10.1523/JNEUROSCI.23-25-08781.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H. The pigment architecture of the human temporal lobe. Anat Embryol. 1978;152:141–169. doi: 10.1007/BF00304663. [DOI] [PubMed] [Google Scholar]
- Bregman AS. Auditory scene analysis. Cambridge, MA: MIT Press; 1990. [Google Scholar]
- Buckner RL, Goodman J, Burock M, Rotte M, Koutstaal W, Schacter D, Rosen B, Dale AM. Functional-anatomic correlates of object priming in humans revealed by rapid presentation event-related fMRI. Neuron. 1998;20:285–296. doi: 10.1016/s0896-6273(00)80456-0. [DOI] [PubMed] [Google Scholar]
- Budd TW, Barry RJ, Gordon E, Rennie C, Michie PT. Decrement of the N1 auditory event-related potential with stimulus repetition: habituation vs. refractoriness. Int J Psychophysiol. 1998;31:51–68. doi: 10.1016/s0167-8760(98)00040-3. [DOI] [PubMed] [Google Scholar]
- Butler RA. Effect of changes in stimulus frequency and intensity on habituation of the human vertex potential. J Acoust Soc Am. 1968;44:945–950. doi: 10.1121/1.1911233. [DOI] [PubMed] [Google Scholar]
- Engel SA, Furmanski CS. Selective adaptation to color contrast in human primary visual cortex. J Neurosci. 2001;21:3949–3954. doi: 10.1523/JNEUROSCI.21-11-03949.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eytan D, Brenner N, Marom S. Selective adaptation in networks of cortical neurons. J Neurosci. 2003;23:9349–9356. doi: 10.1523/JNEUROSCI.23-28-09349.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishbach A, Nelken I, Yeshurun Y. Auditory edge detection: a neural model for physiological and psychoacoustical responses to amplitude transients. J Neurophysiol. 2001;85:2303–2323. doi: 10.1152/jn.2001.85.6.2303. [DOI] [PubMed] [Google Scholar]
- Flanagan JL, Guttman N. On the pitch of periodic pulses. J Acoust Soc Am. 1960;32:1308–1328. [Google Scholar]
- Formisano E, Kim DSK, Di Salle F, van de Moortele PF, Ugurbil K, Goebel R. Mirror-symmetric tonotopic maps in human primary auditory cortex. Neuron. 2003;40:859–869. doi: 10.1016/s0896-6273(03)00669-x. [DOI] [PubMed] [Google Scholar]
- Galaburda A, Sanides F. Cytoarchitectonic organization of the human auditory cortex. J Comp Neurol. 1980;190:597–610. doi: 10.1002/cne.901900312. [DOI] [PubMed] [Google Scholar]
- Griffiths TD, Büchel C, Frackowiak RSJ, Patterson RD. Analysis of temporal structure in sound by the brain. Nat Neurosci. 1998;1:422–427. doi: 10.1038/1637. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Kushnir T, Edelman S, Avidan G, Itzchak Y, Malach R. Differential processing of objects under various viewing conditions in the human lateral occipital complex. Neuron. 1999;24:187–203. doi: 10.1016/s0896-6273(00)80832-6. [DOI] [PubMed] [Google Scholar]
- Gutschalk A, Patterson RD, Rupp A, Uppenkamp S, Scherg M. Sustained magnetic fields reveal separate sites for sound level and temporal regularity in human auditory cortex. Neuroimage. 2002;15:207–216. doi: 10.1006/nimg.2001.0949. [DOI] [PubMed] [Google Scholar]
- Gutschalk A, Patterson RD, Scherg M, Uppenkamp S, Rupp A. Temporal dynamics of pitch in human auditory cortex. Neuroimage. 2004;22:755–766. doi: 10.1016/j.neuroimage.2004.01.025. [DOI] [PubMed] [Google Scholar]
- Gutschalk A, Patterson RD, Uppenkamp S, Scherg M, Rupp A. Recovery and refractoriness of auditory evoked fields after gaps in click trains. Eur J Neurosci. 2004;20:3141–3147. doi: 10.1111/j.1460-9568.2004.03767.x. [DOI] [PubMed] [Google Scholar]
- Harms MP, Melcher JR. Sound repetition rate in the human auditory pathway: representations in the waveshape and amplitude of fMRI activation. J Neurophysiol. 2002;88:1433–1450. doi: 10.1152/jn.2002.88.3.1433. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Picton TW. On and off components in the auditory evoked potentials. Percept Psychophys. 1978;24:391–398. doi: 10.3758/bf03199736. [DOI] [PubMed] [Google Scholar]
- Huk AC, Ress D, Heeger DJ. Neuronal basis of the motion aftereffect reconsidered. Neuron. 2001;32:161–172. doi: 10.1016/s0896-6273(01)00452-4. [DOI] [PubMed] [Google Scholar]
- Imada T, Watanabe M, Mashiko T, Kawakatsu M, Kotani M. The silent period between sounds has a stronger effect than the interstimulus interval on auditory evoked magnetic fields. Electroencephalogr Clin Neurophysiol. 1997;102:37–45. doi: 10.1016/s0013-4694(96)95125-1. [DOI] [PubMed] [Google Scholar]
- Jääskeläinen IP, Ahveninen J, Bonmassar G, Dale AM, Ilmoniemi RJ, Levänen S, Lin FH, May P, Melcher J, Stufflebeam S, Tiitinen H, Belliveau JW. Human posterior auditory cortex gates novel sounds to consciousness. Proc Natl Acad Sci USA. 2004;101:6809–6814. doi: 10.1073/pnas.0303760101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumbholz K, Patterson RD, Seither-Preisler A, Lammertmann C, Lütkenhöner B. Neuromagnetic evidence for a pitch processing center in Heschl’s gyrus. Cereb Cortex. 2003;13:765–772. doi: 10.1093/cercor/13.7.765. [DOI] [PubMed] [Google Scholar]
- Leonard CM, Puranik C, Kuldau JM, Lombardino LJ. Normal variation in the frequency and location of human auditory cortex landmarks. Heschl’s gyrus: where is it? Cereb Cortex. 1998;8:397–406. doi: 10.1093/cercor/8.5.397. [DOI] [PubMed] [Google Scholar]
- Lü ZL, Williamson SJ, Kaufman L. Human auditory primary and association cortex have different lifetimes for activation traces. Brain Res. 1992;572:236–241. doi: 10.1016/0006-8993(92)90475-o. [DOI] [PubMed] [Google Scholar]
- Mäkelä JP, Hari R, Leinonen L. Magnetic responses of the human auditory cortex to noise/square wave transitions. Electroencephalogr Clin Neurophysiol. 1988;69:423–430. doi: 10.1016/0013-4694(88)90064-8. [DOI] [PubMed] [Google Scholar]
- Malone BJ, Semple MN. Effects of auditory stimulus context on the representation of frequency in the gerbil inferior colliculus. J Neurophysiol. 2001;86:1113–1130. doi: 10.1152/jn.2001.86.3.1113. [DOI] [PubMed] [Google Scholar]
- May P, Tiitinen H, Ilmoniemi RJ, Nyman G, Taylor JG, Näätänen R. Frequency change detection in human auditory cortex. J Comput Neurosci. 1999;6:99–120. doi: 10.1023/a:1008896417606. [DOI] [PubMed] [Google Scholar]
- Morel A, Garraghty PE, Kaas JH. Tonotopic organization, architectonic fields, and connections of auditory cortex in macaque monkeys. J Comp Neurol. 1993;273:52–66. doi: 10.1002/cne.903350312. [DOI] [PubMed] [Google Scholar]
- Morosan P, Rademacher J, Schleicher A, Amunts K, Schormann T, Zilles K. Human primary auditory cortex: cytoarchitectonic subdivisions and mapping into a spatial reference system. Neuroimage. 2001;13:684–701. doi: 10.1006/nimg.2000.0715. [DOI] [PubMed] [Google Scholar]
- Movshon JA, Lennie P. Pattern-selective adaptation in visual cortical neurones. Nature. 1979;278:850–853. doi: 10.1038/278850a0. [DOI] [PubMed] [Google Scholar]
- Näätänen R, Picton TW. The N1 wave of the human electric and magnetic response to sound: a review and an analysis of the component structure. Psychophysiology. 1987;24:375–425. doi: 10.1111/j.1469-8986.1987.tb00311.x. [DOI] [PubMed] [Google Scholar]
- Patterson RD. The effects of relative phase and the number of components on residue pitch. J Acoust Soc Am. 1973;53:1565–1572. doi: 10.1121/1.1913504. [DOI] [PubMed] [Google Scholar]
- Patterson RD, Handel S, Yost WA, Datta AJ. The relative strength of the tone and noise components in iterated rippled noise. J Acoust Soc Am. 1996;100:3286–3294. [Google Scholar]
- Patterson RD, Uppenkamp S, Johnsrude IS, Griffiths TD. The processing of temporal pitch and melody information in auditory cortex. Neuron. 2002;36:767–776. doi: 10.1016/s0896-6273(02)01060-7. [DOI] [PubMed] [Google Scholar]
- Penagos H, Melcher JR, Oxenham AJ. A neural representation of pitch salience in non-primary human auditory cortex revealed with fMRI. J Neurosci. 2004;24:6810–6815. doi: 10.1523/JNEUROSCI.0383-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picton TW, Alain C, Otten L, Ritter W, Achim A. Mismatch negativity: different water in the same river. Audiol Neurootol. 2000;5:111–139. doi: 10.1159/000013875. [DOI] [PubMed] [Google Scholar]
- Picton TW, Woods DL, Proulx GB. Human auditory sustained potentials. I. The nature of the response. Electroencephalogr Clin Neurophysiol. 1978a;45:186–197. doi: 10.1016/0013-4694(78)90003-2. [DOI] [PubMed] [Google Scholar]
- Picton TW, Woods DL, Proulx GB. Human auditory sustained potentials. II. Stimulus relationships. Electroencephalogr Clin Neurophysiol. 1978b;45:198–210. doi: 10.1016/0013-4694(78)90004-4. [DOI] [PubMed] [Google Scholar]
- Pollack I. Detection and relative discrimination of auditory “jitter”. J Acoust Soc Am. 1968;43:308–315. doi: 10.1121/1.1910780. [DOI] [PubMed] [Google Scholar]
- Rademacher J, Morosan P, Schormann T, Schleicher A, Werner C, Freund HJ, Zilles K. Probabilistic mapping and volume measurement of human primary auditory cortex. Neuroimage. 2001;13:669–683. doi: 10.1006/nimg.2000.0714. [DOI] [PubMed] [Google Scholar]
- Ritter W, Vaughan HG, Jr, Costa LD. Orienting and habituation to auditory stimuli: a study of short term changes in average evoked responses. Electroencephalogr Clin Neurophysiol. 1968;25:550–556. doi: 10.1016/0013-4694(68)90234-4. [DOI] [PubMed] [Google Scholar]
- Rupp A, Uppenkamp U, Bailes J, Gutschalk A, Patterson RD. Time constants in temporal pitch extraction: a comparison of psychophysical and neuromagnetic data. In: Pressnitzer D, de Chevigne A, McAdams S, Collet L, editors. Auditory signal processing: physiology, psychoacoustics, and models. New York: Springer; 2004. pp. 119–125. [Google Scholar]
- Scherg M. Fundamentals of dipole source analysis. In: Grandori F, Hoke M, Romani GL, editors. Auditory evoked magnetic fields and electric potentials. Vol. 6. Basel, Germany: Karger; 1990. pp. 40–69. (advances in audiology). [Google Scholar]
- Scherg M, Vajsar J, Picton TW. A source analysis of the human auditory evoked potentials. J Cogn Neurosci. 1989;1:336–354. doi: 10.1162/jocn.1989.1.4.336. [DOI] [PubMed] [Google Scholar]
- Talairach P, Tournoux J. A stereotactic coplanar atlas of the human brain. Stuttgart, Germany: Thieme; 1988. [Google Scholar]
- Ulanovsky N, Las L, Farkas D, Nelken I. Multiple time scales of adaptation in auditory cortex neurons. J Neurosci. 2004;24:10440–10453. doi: 10.1523/JNEUROSCI.1905-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulanovsky N, Las L, Nelken I. Processing of low-probability sounds by cortical neurons. Nat Neurosci. 2003;6:391–398. doi: 10.1038/nn1032. [DOI] [PubMed] [Google Scholar]
- Warren JD, Griffiths TD. Distinct mechanisms for processing spatial sequences and pitch sequences in the human auditory brain. J Neurosci. 2003;23:5799–5804. doi: 10.1523/JNEUROSCI.23-13-05799.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yost WA. Pitch strength of iterated rippled noise. J Acoust Soc Am. 1996;100:3329–3335. doi: 10.1121/1.416973. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Bouffard M, Belin P. Sensitivity to auditory object features in human temporal neocortex. J Neurosci. 2004;24:3637–3642. doi: 10.1523/JNEUROSCI.5458-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]