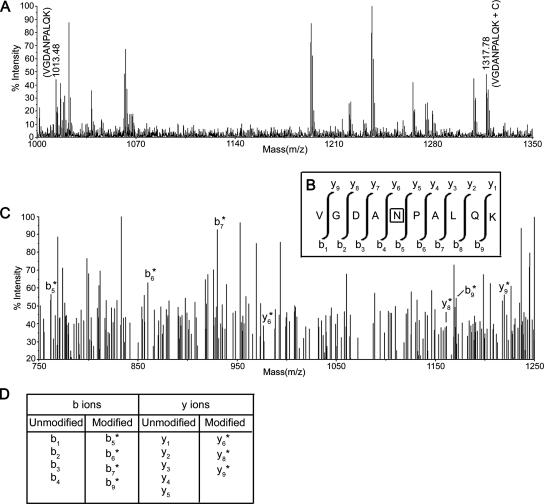
FIG. 3.
MS and MS/MS analysis to show that the peptide stretch 148 VGDANPALQK 157 of BCA remains cross-linked with one cytosine residue of domain V RNA through its Asn152 residue. (A) MS spectrum of the signal at m/z 1317.78, derived after addition with a cytosine (C) residue (molecular weight, 305) from the “parent ion” at m/z 1013.48 (VGDANPALQK) (M+H)+. (B) The sequence of the corresponding peptide, with a scheme of the y and b fragmentation series. The asparagine residue within the box is cross-linked with the C moiety. (C) MS/MS spectrum of modified VGDANPALQK (m/z = 1317.78, i.e., attached with C) recorded with a 2,5-dihydroxy benzoic acid matrix. The spectra show an almost complete series of y-type and b-type fragments (b5/y5 to b9/y9 shown). Ion b5 is attached with a C residue, as revealed by the m/z value, while y6 is also attached with a C residue, so Asn152 is cross-linked with the C residue of domain V. (D) Complete list of b and y ions is presented here. b1 to b4 and y1 to y5 indicate unmodified ions. b5* to b9* and y6* to y9* represent modified ions attached with a C residue (the asterisk is used to depict modified ions).