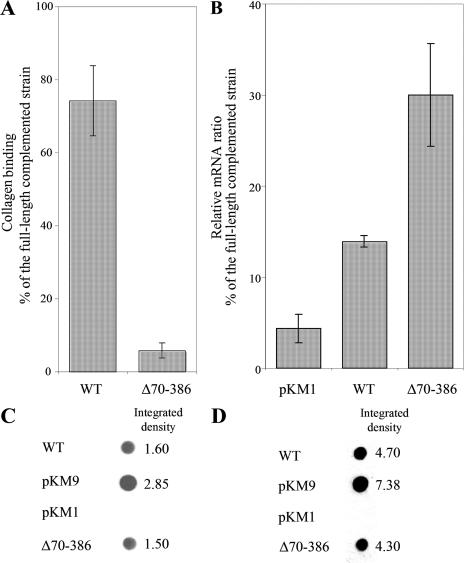
FIG. 2.
(A) Collagen binding activity of the wild-type (WT) strain VT1169 and the emaA mutant strain complemented with pKMΔ70-386 (plasmid containing an in-frame deletion corresponding to amino acids 70 to 386) as measured by enzyme-linked immunosorbent assay (ELISA). The data are represented as the percentage of the binding of the emaA mutant strain complemented with the full-length sequence (pKM9). (B) Quantitative real-time PCR for emaA of RNA isolated from the wild type or the emaA mutant strains transformed with the empty plasmid (pKM1) or pKMΔ70-386. The results are expressed as ratios of the emaA RNA from the emaA mutant strain transformed with the full-length sequence (pKM9). (C) Immunodot blot of whole-cell lysates from 2 × 108 CFU of bacterial preparations. EmaA was detected by using a monoclonal antibody specific for amino acids 631 to 1355. The integrated density of each dot was quantified by using the software ImageJ. (D) Immunodot blot of 5 μg of membrane proteins from each strain.