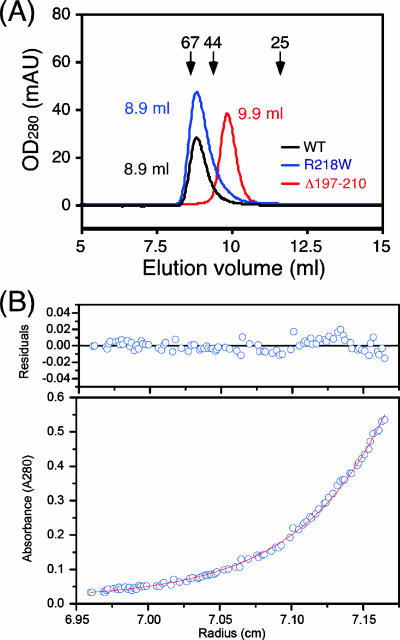
FIG. 5.
Hydrodynamic properties of MotBC mutant proteins. (A) Elution profiles of purified MotBC-His6 fragments determined by analytical size exclusion chromatography with a Superdex 75 HR 10/30 column. Black line, wild-type (WT) MotBC (peak at 8.9 ml); blue line, MotBC(R218W) mutant protein (peak at 8.9 ml); red line, MotBC(Δ197-210) mutant protein (peak at 9.9 ml). Arrows indicate the elution peaks of the size marker proteins BSA (67 kDa), ovalbumin (44 kDa), and chymotrypsinogen (25 kDa) at 8.6 ml, 9.4 ml, 11.6 ml, respectively. OD280, optical density at 280 nm; mAU, milliabsorbance units. (B) Sedimentation equilibrium analytical ultracentrifugation of MotBC(Δ197-210)-His6. Open circles are data points, and the continuous line is a model fit. A concentration profile for MotBC(Δ197-210)-His6 (initial absorbance at 280 nm of 0.2, measured at 28,000 rpm) is shown at the bottom. For data fitting, we performed a global fit to six data sets from two different protein concentrations and three rotor speeds. The residuals due to deviation of the data from this line are shown at the top. The molecular mass obtained was 24.0 kDa, indicating that MotBC(Δ197-210)-His6 is a monomer. Measurements were done at room temperature.