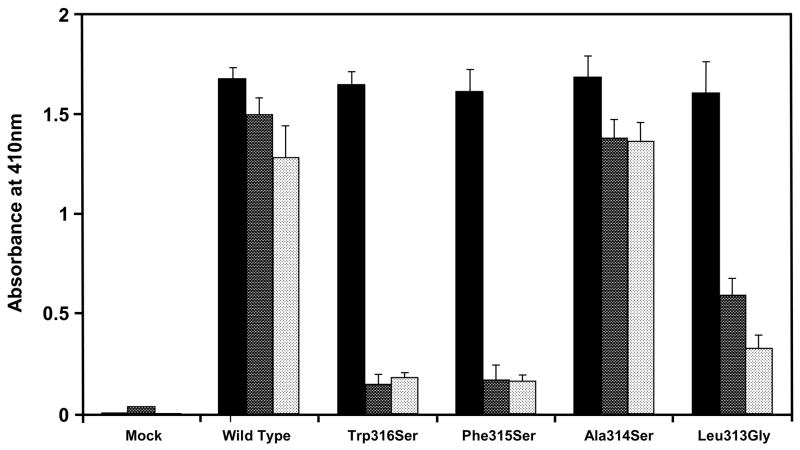
Fig. 2.
Effect of hydrophobic amino acids in the fifth domain of rβ2GPI at positions 313–316 on its ability to bind to rHBsAg and CL. Four hydrophobic amino acids at positions 313–316 were mutated to neutral amino acids (Trp316Ser, Phe315Ser, Ala314Ser and Leu313Gly) and expressed in COS-1 cells. The secreted rβ2GPI forms were quantitated by capture ELISA (■) followed by their binding to rHBsAg (
 ) or CL (
) or CL (
 ). Results are given as mean ± SD from three independent clones in triplicate (n=9).
). Results are given as mean ± SD from three independent clones in triplicate (n=9).