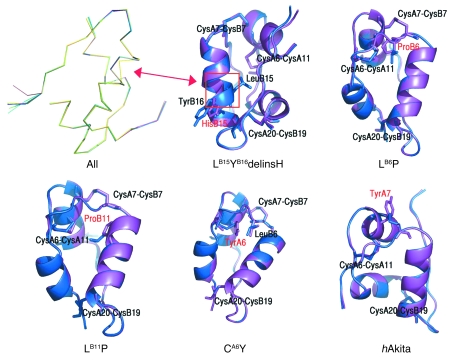
Figure 3. Secondary structure computer modeling of WT and mutant proinsulins.
Shown are superimposed Cα traces of WT (blue) and all mutated insulins (purple), with the exception of LB6V. The positions of disulfide bridges are also marked. None of the mutations caused substantial distortion of the secondary structure, with the exception of LB15YB16delinsH (yellow trace at left), where an α-helical disruption is apparent (arrow). This was also evident in the superimposition of WT insulin and LB15YB16delinsH. The LB6P mutation alters the hydrophobic core of the protein. The LB11P mutation affects the hydrophobic core of the protein. The CA6Y mutation disrupts the A6–A11 disulfide bridge; the tyrosine is oriented inside the hydrophobic core of the protein, where it engages LB6 in a stacking interaction. The hAkita mutation — used in this study as positive control — disrupts the B7–A7 disulfide bridge, and the tyrosine is solvent exposed.