Figure 5.
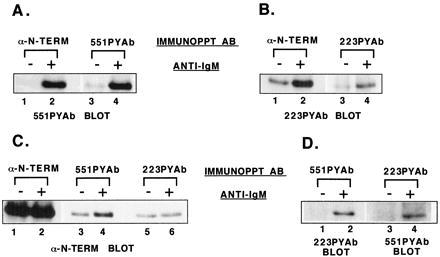
Analysis of the fraction of Btk molecules phosphorylated and the pattern of tyrosine phosphorylation. Ramos B cells were untreated or stimulated with goat anti-human IgM for 1 min. Btk protein was immunoprecipitated from cell extracts with anti-Btk N-terminal antibody, then resolubilized and diluted. Btk from untreated or stimulated samples was immunoprecipitated with 551PYAb, 223PYAb, or preimmune serum. The remaining soluble fraction of Btk then was recovered by immunoprecipitation with anti-Btk N-terminal antibody. Immunoblot analysis was performed as indicated. (A) Comparison of the amount of site 1-phosphorylated Btk recovered from untreated or stimulated cell samples by immunoprecipitation with anti-Btk N-terminal or 551PYAb. (B) Comparison of the amount of site 2-phosphorylated Btk recovered from untreated or stimulated cell samples by immunoprecipitation with anti-Btk N-terminal or 223PYAb. (C) Comparison of the amount of Btk recovered from untreated or stimulated cell samples by immunoprecipitation with 551PYAb, 223PYAb, or anti-Btk N-terminal antibody. (D) Double phosphorylation of Btk molecules recovered from untreated or stimulated cell samples by immunoprecipitation with 551PYAb or 223PYAb, detected by immunoblot analysis with the alternative phosphopeptide-specific antibody.
