Abstract
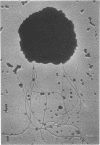
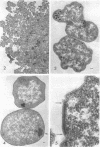
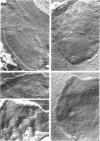
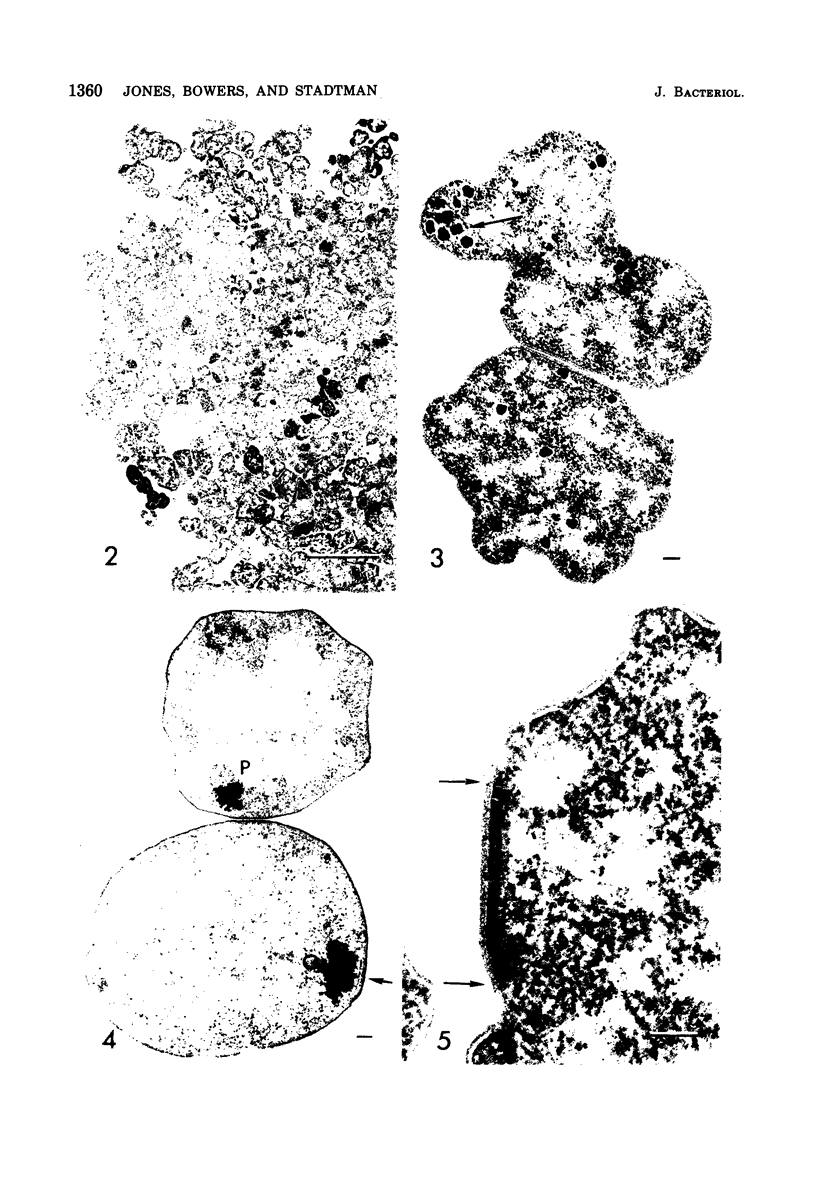
Methanococcus vannielii is a strictly anaerobic motile coccus that possesses a tuft of flagellae. The cells are markedly sensitive to mechanical stress and are readily lysed by detergents, but the organism grows normally in media of low ionic strength. The absence of a typical cell wall, further suggested by resistance of M. vannielii to penicillin, cycloserine, and vancomycin, was confirmed by ultrastructural studies. Electron micrographs showed that the cell envelope lacks a peptidoglycan layer. On the outer surface there is a regular array of subunits similar to those of the glycoprotein envelopes of the halobacteria. However, the M. vannielii cell envelope, unlike those of the holobacteria, is unable to maintain a definite shape, and a high salt concentration is not required for its integrity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gilpin R. W., Young F. E., Chatterjee A. N. Characterization of a stable L-form of Bacillus subtilis 168. J Bacteriol. 1973 Jan;113(1):486–499. doi: 10.1128/jb.113.1.486-499.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUSHNER D. J., BAYLEY S. T., BORING J., KATES M., GIBBONS N. E. MORPHOLOGICAL AND CHEMICAL PROPERTIES OF CELL ENVELOPES OF THE EXTREME HALOPHILE, HALOBACTERIUM CUTIRUBRUM. Can J Microbiol. 1964 Jun;10:483–497. doi: 10.1139/m64-058. [DOI] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mescher M. F., Strominger J. L. Purification and characterization of a prokaryotic glucoprotein from the cell envelope of Halobacterium salinarium. J Biol Chem. 1976 Apr 10;251(7):2005–2014. [PubMed] [Google Scholar]
- Mescher M. F., Strominger J. L., Watson S. W. Protein and carbohydrate composition of the cell envelope of Halobacterium salinarium. J Bacteriol. 1974 Nov;120(2):945–954. doi: 10.1128/jb.120.2.945-954.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEUHAUS F. C., LYNCH J. L. THE ENZYMATIC SYNTHESIS OF D-ALANYL-D-ALANINE. 3. ON THE INHIBITION OF D-ALANYL-D-ALANINE SYNTHETASE BY THE ANTIBIOTIC D-CYCLOSERINE. Biochemistry. 1964 Apr;3:471–480. doi: 10.1021/bi00892a001. [DOI] [PubMed] [Google Scholar]
- PARK J. T., HANCOCK R. A fractionation procedure for studies of the synthesis of cell-wall mucopeptide and of other polymers in cells of Staphylococcus aureus. J Gen Microbiol. 1960 Feb;22:249–258. doi: 10.1099/00221287-22-1-249. [DOI] [PubMed] [Google Scholar]
- Reusch V. M., Panos C. Defective synthesis of lipid intermediates for peptidoglycan formation in a stabilized L-form of Streptococcus pyogenes. J Bacteriol. 1976 Apr;126(1):300–311. doi: 10.1128/jb.126.1.300-311.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STADTMAN T. C., BARKER H. A. Studies on the methane fermentation. X. A new formate-decomposing bacterium, Methanococcus vannielii. J Bacteriol. 1951 Sep;62(3):269–280. doi: 10.1128/jb.62.3.269-280.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Steensland H., Larsen H. A study of the cell envelope of the halobacteria. J Gen Microbiol. 1969 Mar;55(3):325–336. doi: 10.1099/00221287-55-3-325. [DOI] [PubMed] [Google Scholar]
- Stoeckenius W., Rowen R. A morphological study of Halobacterium halobium and its lysis in media of low salt concentration. J Cell Biol. 1967 Jul;34(1):365–393. doi: 10.1083/jcb.34.1.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone K. J., Strominger J. L. Mechanism of action of bacitracin: complexation with metal ion and C 55 -isoprenyl pyrophosphate. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3223–3227. doi: 10.1073/pnas.68.12.3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne K. J., Oliver R. C., Glauert A. M. Synthesis and turnover of the regularly arranged surface protein of Acinetobacter sp. relative to the other components of the cell envelope. J Bacteriol. 1976 Jul;127(1):440–450. doi: 10.1128/jb.127.1.440-450.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornley M. J. Cell envelopes with regularly arranged surface subunits in Acinetobacter and related bacteria. CRC Crit Rev Microbiol. 1975 Oct;4(1):65–100. doi: 10.3109/10408417509105487. [DOI] [PubMed] [Google Scholar]
- Tipper D. J., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. XII. Inhibition of cross-linking by penicillins and cephalosporins: studies in Staphylococcus aureus in vivo. J Biol Chem. 1968 Jun 10;243(11):3169–3179. [PubMed] [Google Scholar]
- Weiss R. L. Subunit cell wall of Sulfolobus acidocaldarius. J Bacteriol. 1974 Apr;118(1):275–284. doi: 10.1128/jb.118.1.275-284.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]