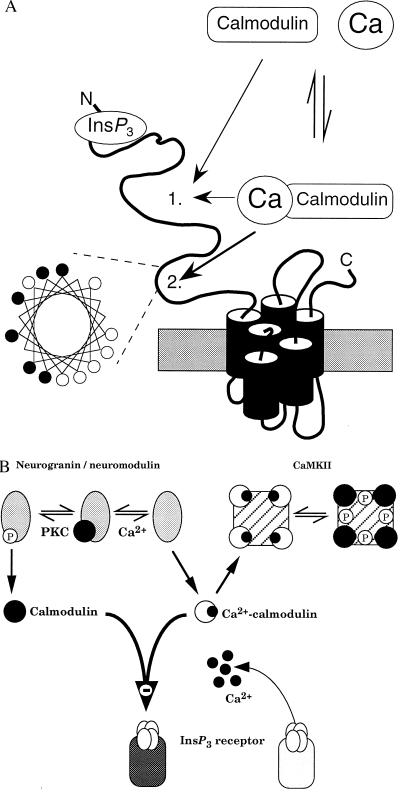
Figure 6.
Interactions between calmodulin and neuronal InsP3 receptors. (A) Predicted structure of a single subunit of the type 1 InsP3 receptor. Our results establish that both calmodulin and Ca2+–calmodulin bind with the same affinity to a site on the InsP3 receptor to decrease its affinity for InsP3; the exact location of this site 1 is unknown. Another calmodulin-binding site (site 2) within the modulatory domain of the receptor binds only Ca2+–calmodulin (27) and, as the helical wheel representation demonstrates, that site has the basic amphipathic helical structure found in other Ca2+–calmodulin-binding proteins (42). Within the helical wheel, basic residues (Arg, His, Lys) are denoted by •, and hydrophobic residues (Ile, Ala, Trp, Val, Leu) by ○. (B) Both neurogranin (postsynaptic) and neuromodulin (presynaptic) are exclusively neuronal and release their bound calmodulin after either an increase in cytosolic Ca2+ concentration or phosphorylation by protein kinase C (PKC). CaMKII binds Ca2+–calmodulin, which triggers autophosphorylation causing the calmodulin to remain bound after the Ca2+ concentration has returned to its resting level. The ensuing changes in cytosolic calmodulin concentration will regulate binding of InsP3 to its receptor irrespective of the prevailing cytosolic Ca2+ concentration.