Abstract
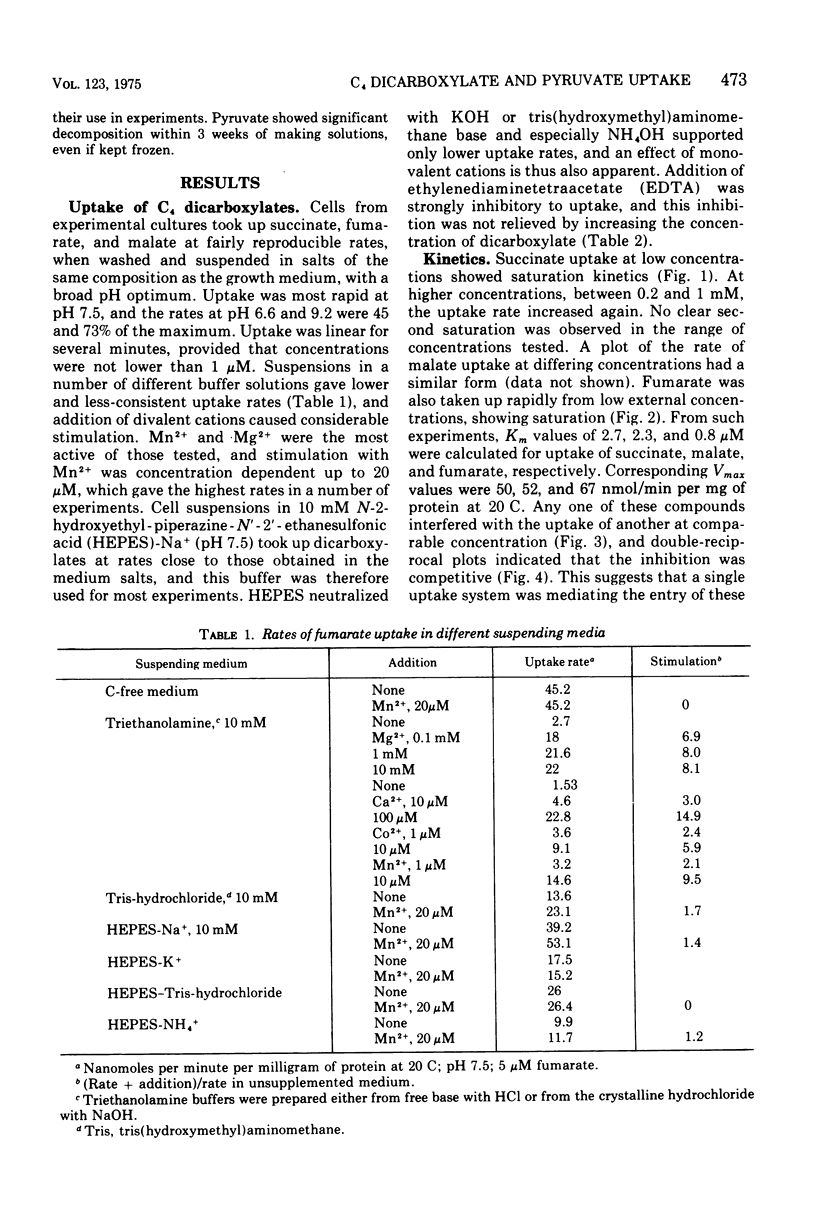
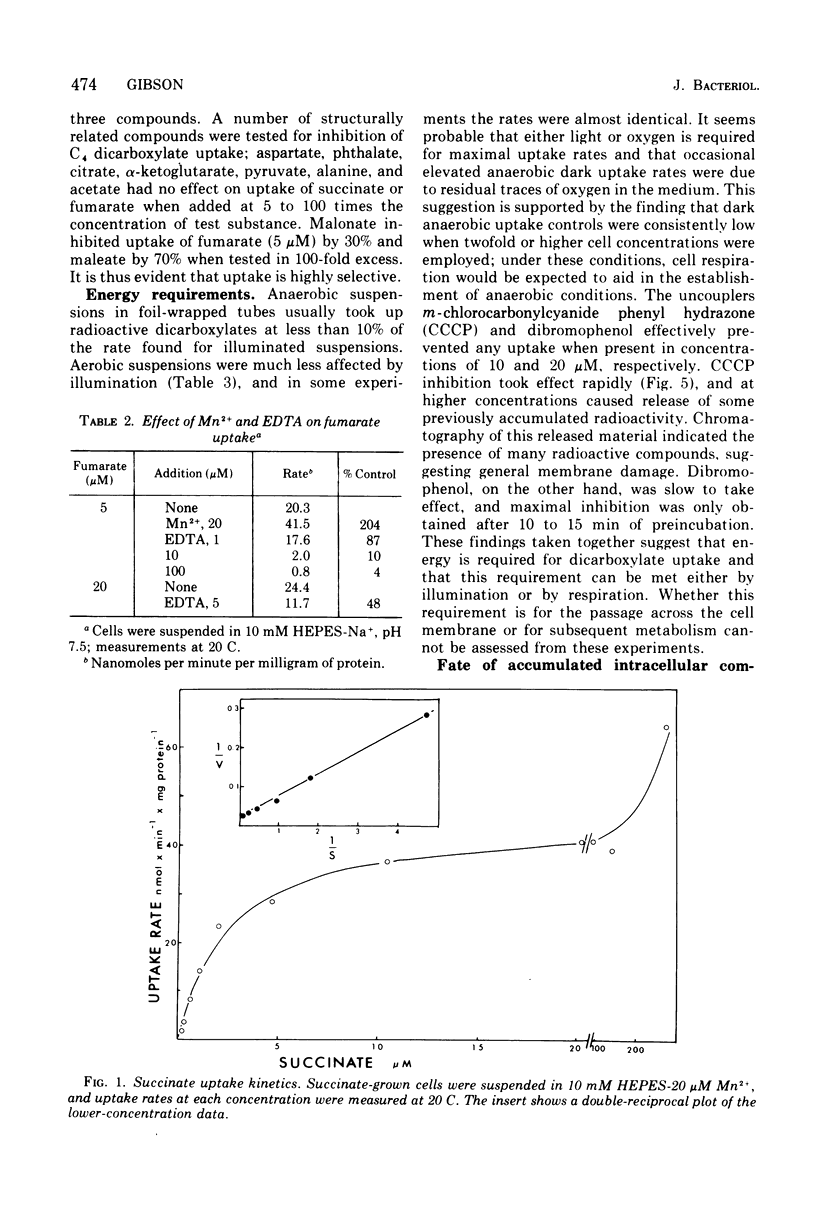
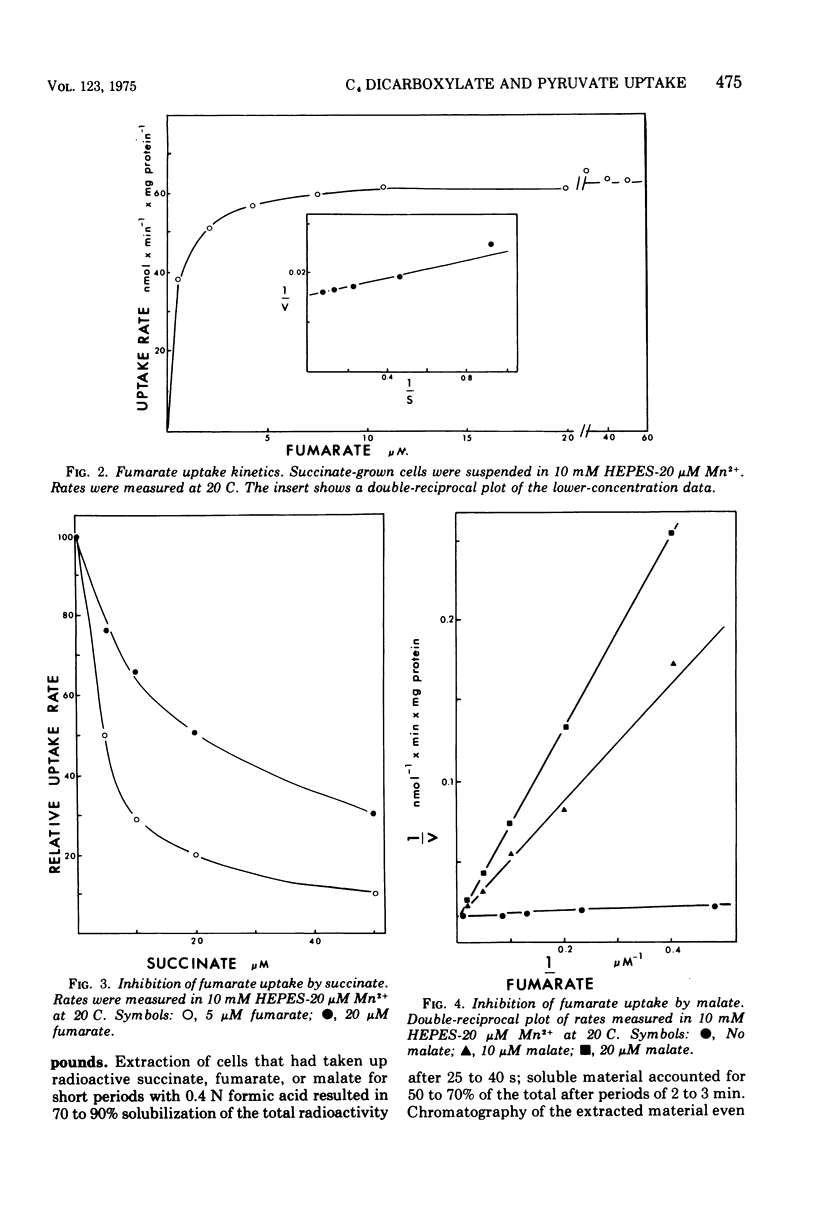
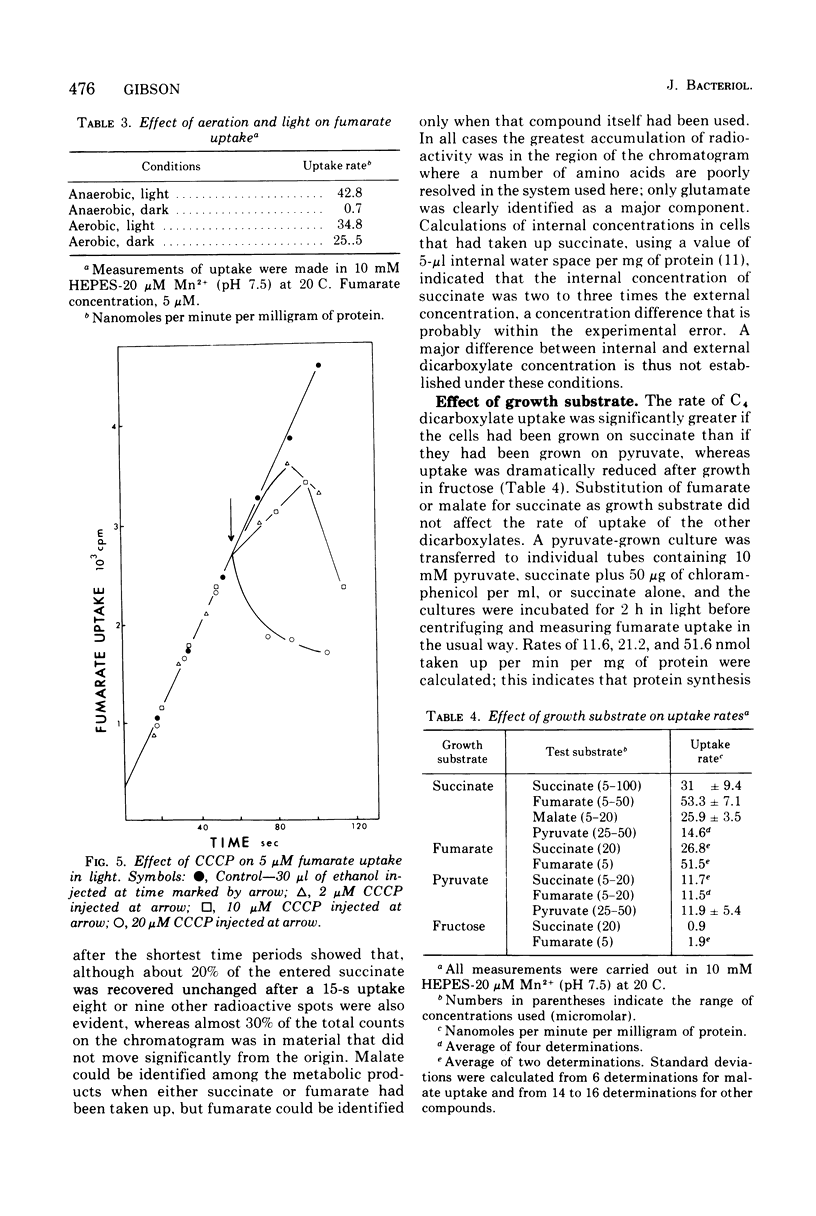
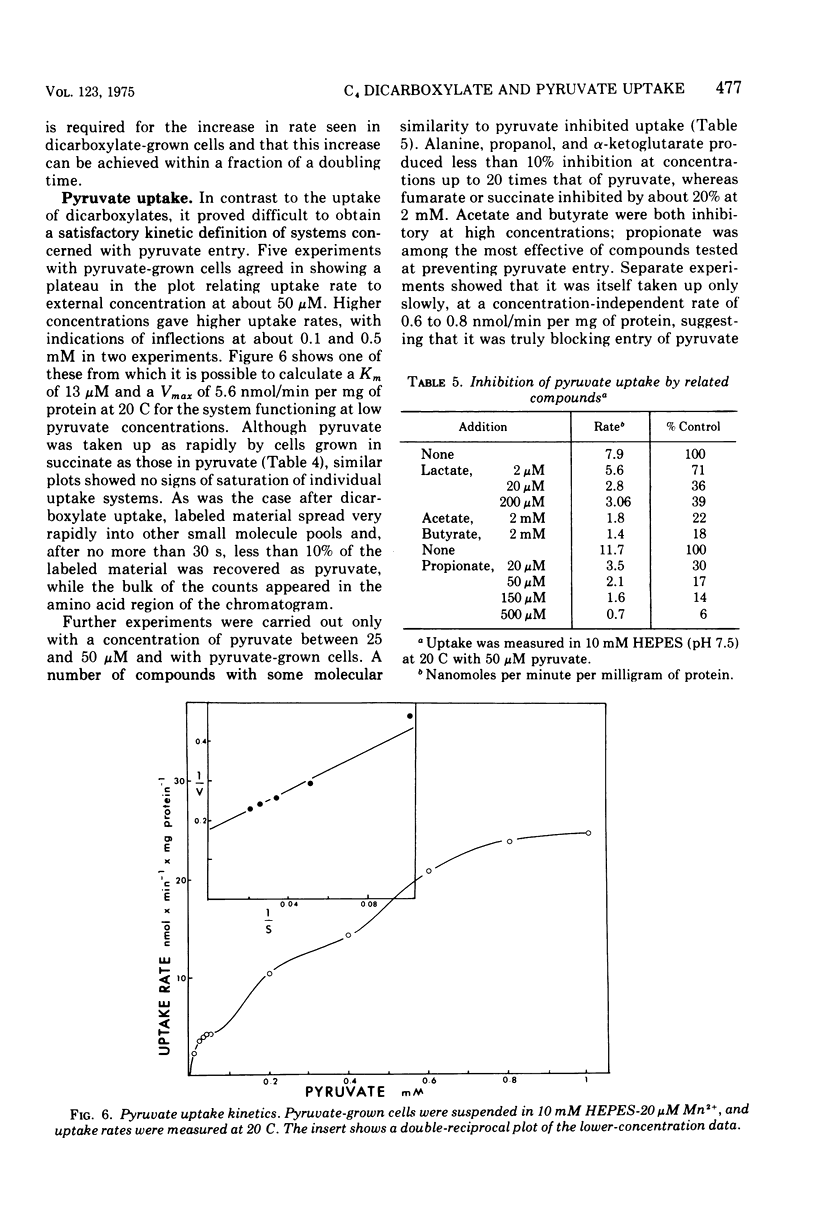
The uptake of C4 dicarboxylates by cells from exponential cultures of Rhodopseudomonas spheroides followed saturation kinetics at concentrations below 100 muM with Km values for succinate, malate, and fumarate of 2.7, 2.3, and 0.8, respectively. Corresponding Vmax values of 50, 52, and 67.5 nmol/min per mg of protein at 20 C were obtained. Each of these compounds interfered competitively with uptake of the others, and a common transport system appears to be involved. Fructose-grown cells took up C4 dicarboxylates only at very low rates, and pyruvate-grown cells took up C4 dicarboxylates at one-third the rates found with succinate-grown cultures. Malonate and maleate inhibited uptake less severely, and aspartate and alpha-ketoglutarate had no effect at 100-fold excess. Divalent metals stimulated uptake. Light or respiration was required for uptake, and entered materials were rapidly converted to other metabolities, notably amino acids. Pyruvate entry appeared to be mediated by several systems, of which only one could be resolved kinetically. This system had a Km of 13 muM and Vmax of 5.6 nmol/min per mg of protein at 20 C. A number of related mono- and dicarboxylates interfered with pyruvate uptake. The pyruvate uptake system was distinguishable from the C4 dicarboxylate system by the absence of divalent cation stimulation and by substrate and inhibitor specificity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fournier R. E., McKillen M. N., Pardee A. B., Willecke K. Transport of dicarboxylic acids in Bacillus subtilis. Inducible uptake of L-malate. J Biol Chem. 1972 Sep 10;247(17):5587–5595. [PubMed] [Google Scholar]
- Frerman F. E. The role of acetyl coenzyme A: butyrate coenzyme A in the transferase uptake of butyrate by isolated membrane vesicles of Escherichia coli. Arch Biochem Biophys. 1973 Nov;159(1):444–452. doi: 10.1016/0003-9861(73)90472-4. [DOI] [PubMed] [Google Scholar]
- Ghei O. K., Kay W. W. Properties of an inducible C 4 -dicarboxylic acid transport system in Bacillus subtilis. J Bacteriol. 1973 Apr;114(1):65–79. doi: 10.1128/jb.114.1.65-79.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghei Om K., Kay William W. A Dicarboxyclic acid transport system in Bacillus subtilis. FEBS Lett. 1972 Feb 1;20(2):137–140. doi: 10.1016/0014-5793(72)80777-4. [DOI] [PubMed] [Google Scholar]
- Harold F. M., Levin E. Lactic acid translocation: terminal step in glycolysis by Streptococcus faecalis. J Bacteriol. 1974 Mar;117(3):1141–1148. doi: 10.1128/jb.117.3.1141-1148.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay W. W., Kornberg H. L. The uptake of C4-dicarboxylic acids by Escherichia coli. Eur J Biochem. 1971 Jan;18(2):274–281. doi: 10.1111/j.1432-1033.1971.tb01240.x. [DOI] [PubMed] [Google Scholar]
- LASCELLES J., RODGERS K. The intracellular concentration of succinic acid in photosynthetic bacteria. Biochem J. 1961 Aug;80:244–245. doi: 10.1042/bj0800244. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lo T. C., Rayman M. K., Sanwal B. D. Transport of succinate in Escherichia coli. I. Biochemical and genetic studies of transport in whole cells. J Biol Chem. 1972 Oct 10;247(19):6323–6331. [PubMed] [Google Scholar]
- Matin A., Konings W. N. Transport of lactate and succinate by membrane vesicles of Escherichia coli, Bacillus subtilis and a pseudomonas species. Eur J Biochem. 1973 Apr 2;34(1):58–67. doi: 10.1111/j.1432-1033.1973.tb02728.x. [DOI] [PubMed] [Google Scholar]
- Miović M. L., Gibson J. Nucleotide pools and adenylate energy charge in balanced and unbalanced growth of Chromatium. J Bacteriol. 1973 Apr;114(1):86–95. doi: 10.1128/jb.114.1.86-95.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers W. F., Huang K. Y. Separation of intermediates of the citric acid cycle and related compounds by thin-layer chromatography. Anal Biochem. 1966 Nov;17(2):210–213. doi: 10.1016/0003-2697(66)90199-0. [DOI] [PubMed] [Google Scholar]
- Pfennig N. Photosynthetic bacteria. Annu Rev Microbiol. 1967;21:285–324. doi: 10.1146/annurev.mi.21.100167.001441. [DOI] [PubMed] [Google Scholar]
- Rayman M. K., Lo T. C., Sanwal B. D. Transport of succinate in Escherichia coli. II. Characteristics of uptake and energy coupling with transport in membrane preparations. J Biol Chem. 1972 Oct 10;247(19):6332–6339. [PubMed] [Google Scholar]
- Saier M. H., Jr, Feucht B. U., Roseman S. Phosphoenolpyruvate-dependent fructose phosphorylation in photosynthetic bacteria. J Biol Chem. 1971 Dec 25;246(24):7819–7821. [PubMed] [Google Scholar]
- Wagner B. J., Miović M. L., Gibson J. Utilization of amino acids by Chromatium sp. strain D. Arch Mikrobiol. 1973 Jun 6;91(3):255–272. doi: 10.1007/BF00408912. [DOI] [PubMed] [Google Scholar]
- Willecke K., Lange R. C4-dicarboxylate transport in Bacillus subtilis studied with 3-fluoro-L-erythro-malate as a substrate. J Bacteriol. 1974 Feb;117(2):373–378. doi: 10.1128/jb.117.2.373-378.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Niel C. B. THE CULTURE, GENERAL PHYSIOLOGY, MORPHOLOGY, AND CLASSIFICATION OF THE NON-SULFUR PURPLE AND BROWN BACTERIA. Bacteriol Rev. 1944 Mar;8(1):1–118. doi: 10.1128/br.8.1.1-118.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]


