Abstract
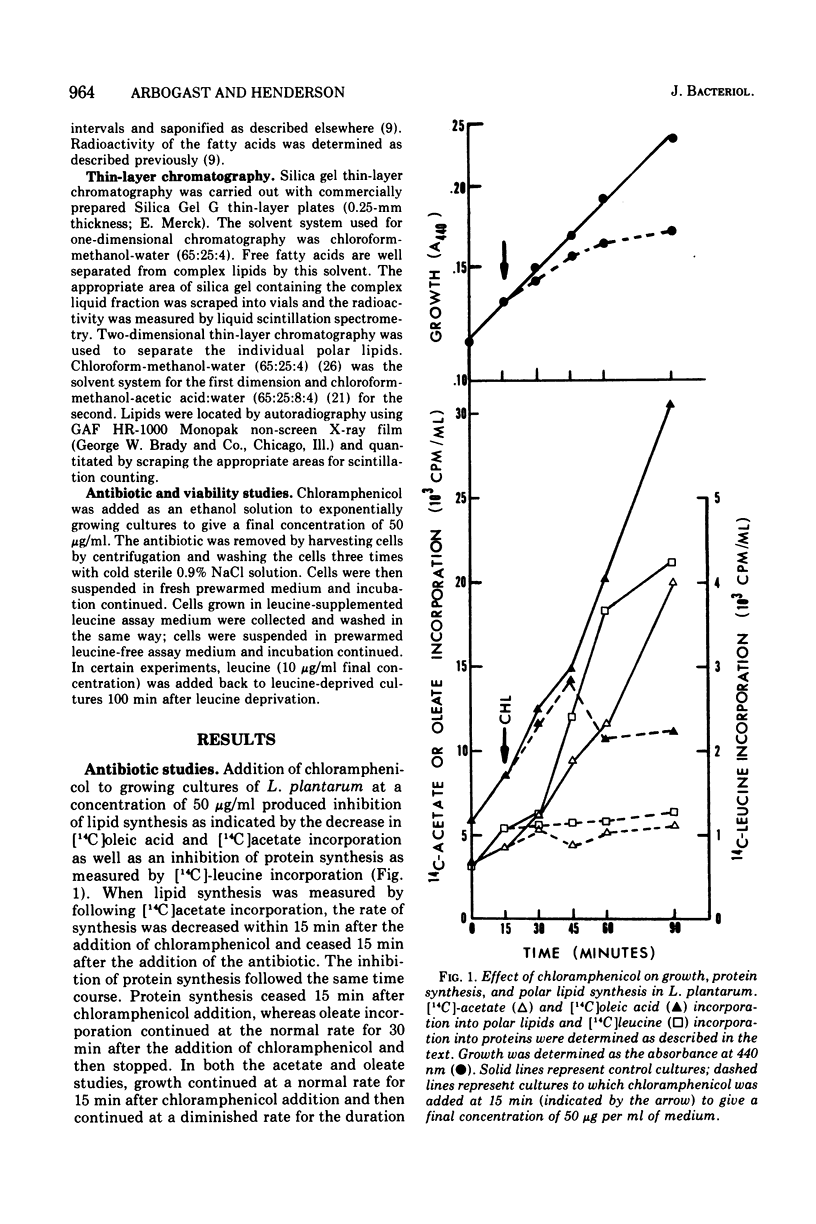
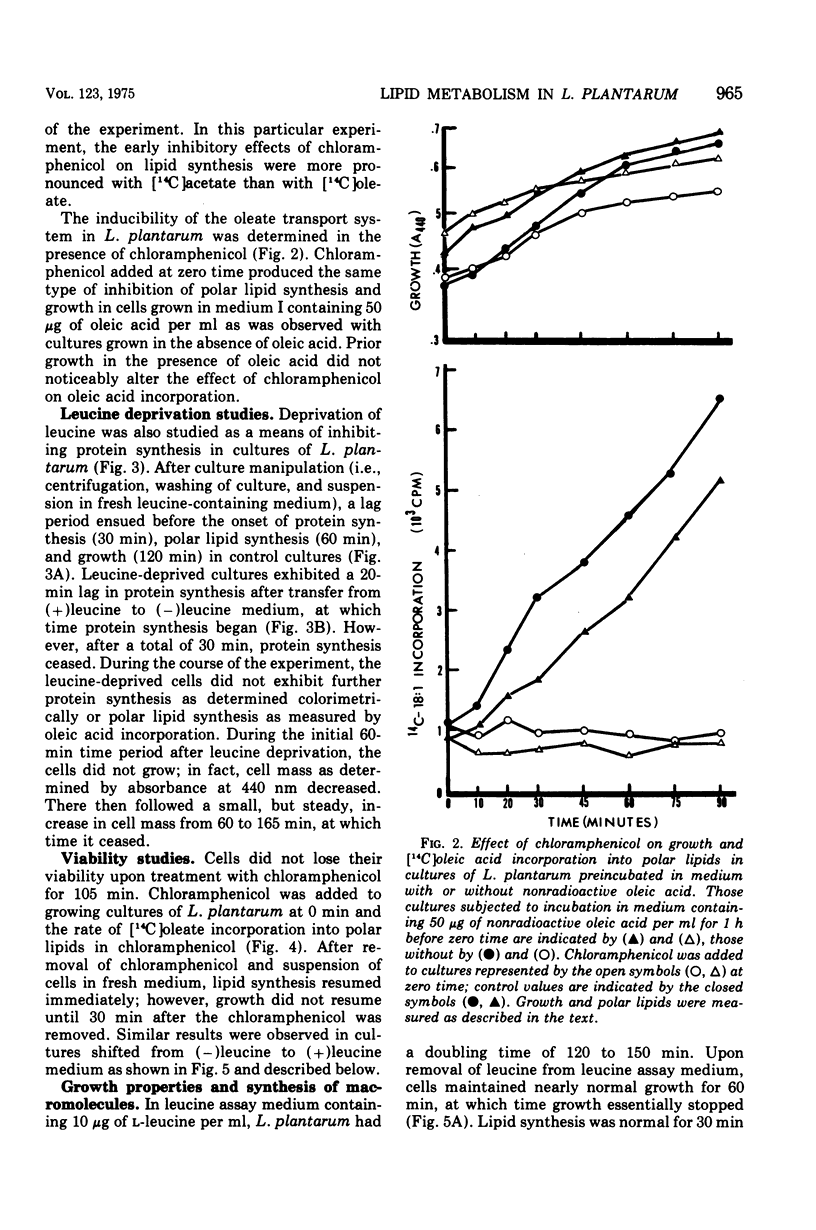
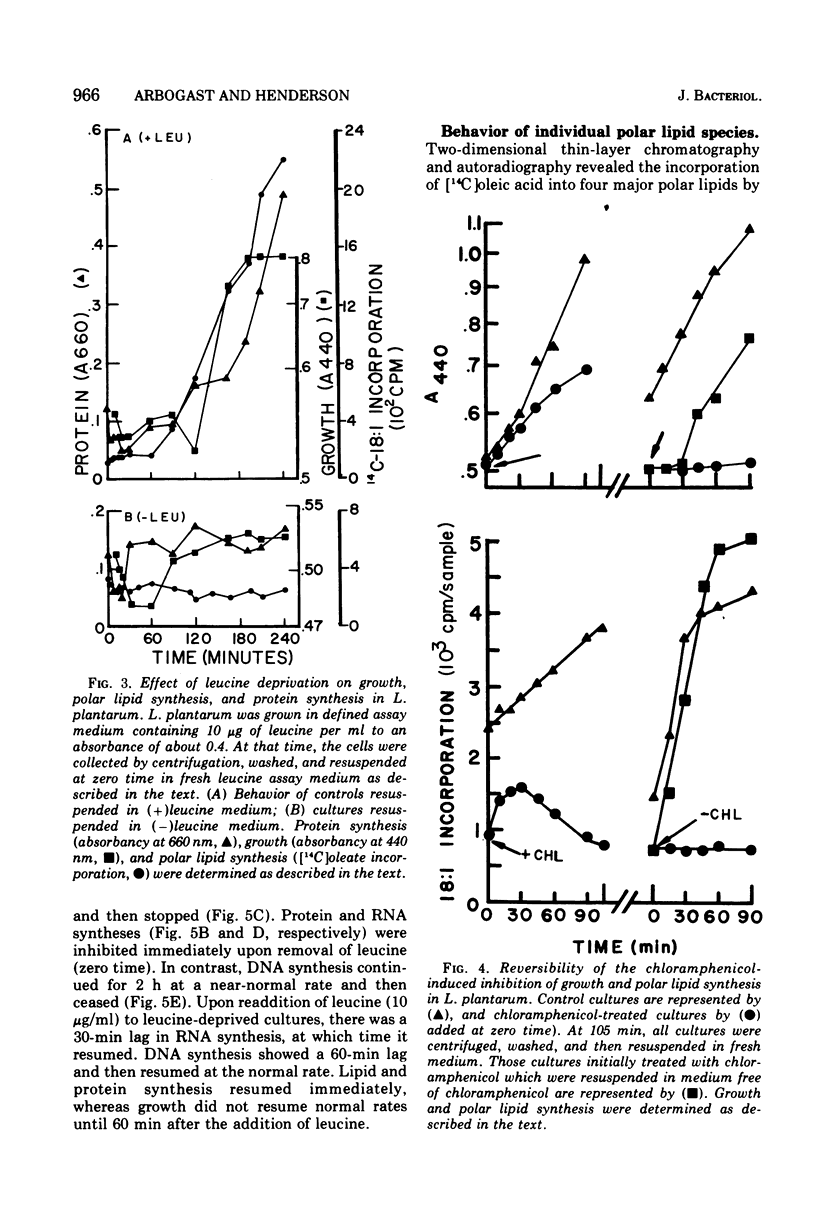
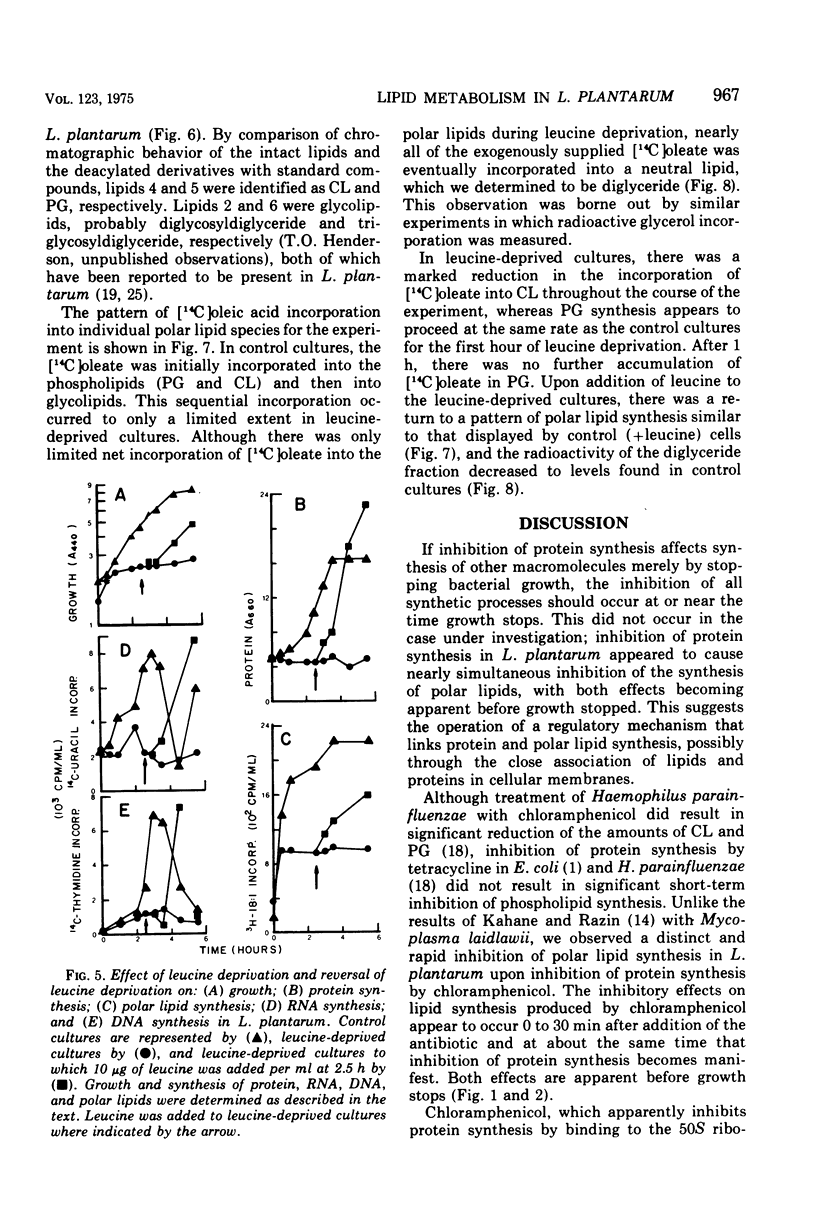
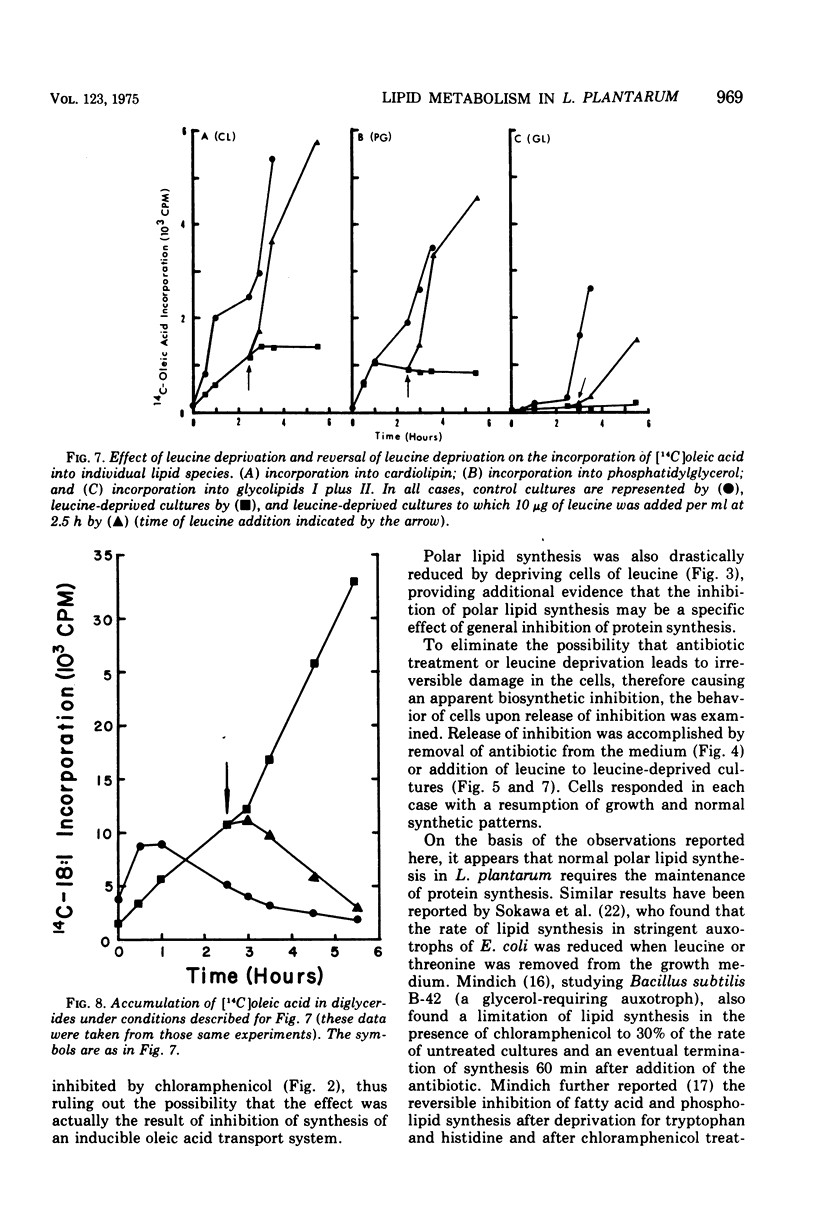
In Lactobacillus plantarum 17-5, lipid synthesis appears to be correlated with protein synthesis. Inhibition of protein synthesis by chloramphenicol (50 mug/ml) caused the nearly simultaneous inhibition of incorporation of radioactive oleic acid into polar lipids before the cessation of growth. In addition, de novo fatty acid synthesis, as determined by the incorporation of radioactive acetate into cellular lipids, was also inhibited. Removal of the antibiotic resulted in the resumption of growth, protein synthesis, and polar lipid synthesis. Inhibition of protein synthesis by leucine deprivation also produced a marked reduction in the incorporation of radioactive oleic acid into the total polar lipids at about the same time that growth stopped (30 to 60 min after the removal of leucine). However, the different classes of lipids behaved differently. For example, the incorporation of oleic acid into cardiolipin was inhibited immediately upon removal of leucine from the cultures, whereas incorporation into phosphatidyl-glycerol was maintained at near normal rates for 60 min after the removal of leucine and then ceased. In contrast, the accumulation of radioactive oleic acid in a neutral lipid identified as diglyceride occurred to a much greater extent in leucine-deprived cultures than in control (+ leucine) cultures. Upon addition of leucine to leucine-deprived cultures, the rates of synthesis of phosphatidyl-glycerol and cardiolipin returned to normal; the amount of radioactivity in the diglyceride fraction decreased to normal levels concomitantly with increased phospholipid synthesis.
Full text
PDF

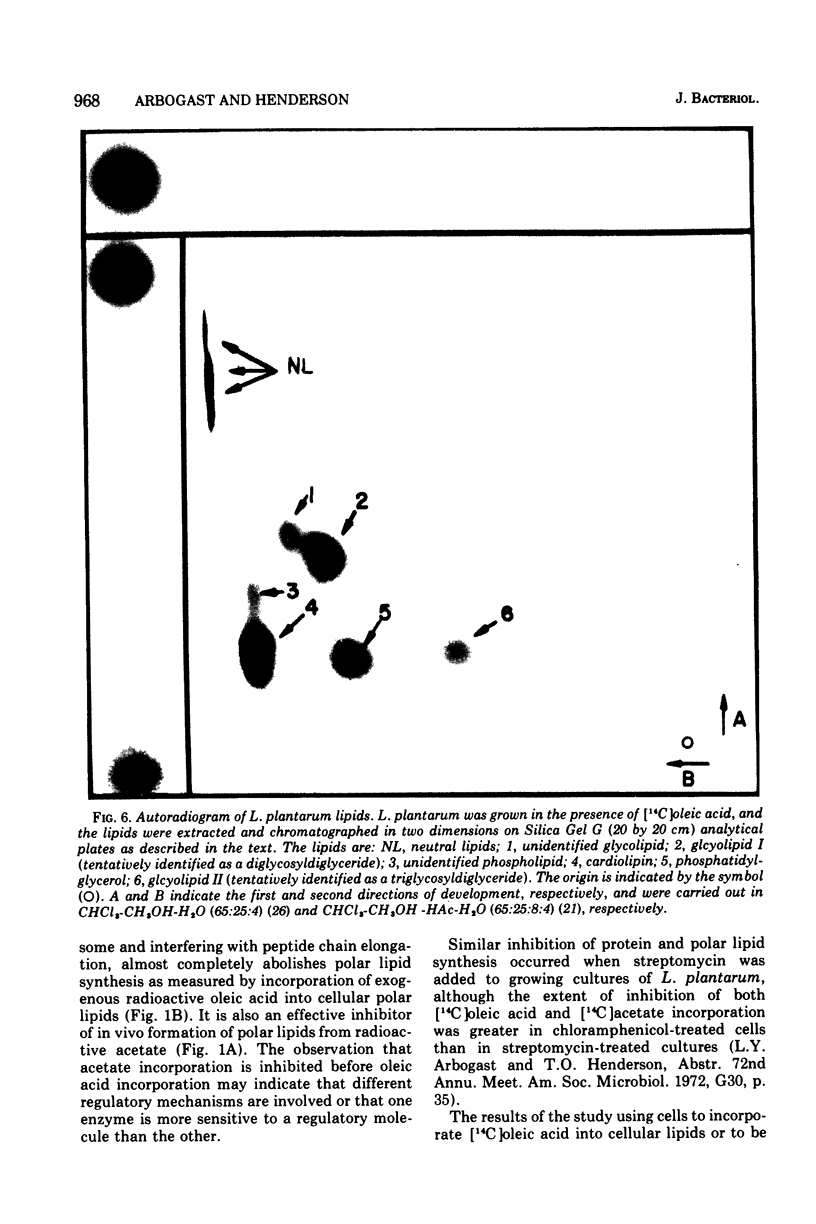


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballesta J. P., Schaechter M. Effect of shift-down and growth inhibition on phospholipid metabolism of Escherichia coli. J Bacteriol. 1971 Jul;107(1):251–258. doi: 10.1128/jb.107.1.251-258.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burritt M. F., Henderson T. O. Properties of a membrane-bound cardiolipin synthetase from Lactobacillus plantarum. J Bacteriol. 1975 Sep;123(3):972–977. doi: 10.1128/jb.123.3.972-977.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan J. E., Vagelos P. R. Metabolism and function of the membrane phospholipids of Escherichia coli. Biochim Biophys Acta. 1972 Feb 14;265(1):25–60. doi: 10.1016/0304-4157(72)90018-4. [DOI] [PubMed] [Google Scholar]
- Exterkate F. A., Otten B. J., Wassenberg H. W., Veerkamp J. H. Comparison of the phospholipid composition of Bifidobacterium and Lactobacillus strains. J Bacteriol. 1971 Jun;106(3):824–829. doi: 10.1128/jb.106.3.824-829.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox C. F. A lipid requirement for induction of lactose transport in Escherichia coli. Proc Natl Acad Sci U S A. 1969 Jul;63(3):850–855. doi: 10.1073/pnas.63.3.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDFINE H., ELLIS M. E. N-METHYL GROUPS IN BACTERIAL LIPIDS. J Bacteriol. 1964 Jan;87:8–15. doi: 10.1128/jb.87.1.8-15.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser M., Bayer W. H., Bell R. M., Vagelos P. R. Regulation of macromolecular biosynthesis in a mutant of Escherichia coli defective in membrane phospholipid biosynthesis. Proc Natl Acad Sci U S A. 1973 Feb;70(2):385–389. doi: 10.1073/pnas.70.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning U., Dennert G., Rehn K., Deppe G. Effects of oleate starvation in a fatty acid auxotroph of Escherichia coli K-12. J Bacteriol. 1969 May;98(2):784–796. doi: 10.1128/jb.98.2.784-796.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtsmuller U. M., van Deenen L. L. On the amino acid esters of phosphatidyl glycerol from bacteria. Biochim Biophys Acta. 1965 Dec 2;106(3):564–576. doi: 10.1016/0005-2760(65)90072-x. [DOI] [PubMed] [Google Scholar]
- IKAWA M. NATURE OF THE LIPIDS OF SOME LACTIC ACID BACTERIA. J Bacteriol. 1963 Apr;85:772–781. doi: 10.1128/jb.85.4.772-781.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahane I., Razin S. Synthesis and turnover of membrane protein and lipid in Mycoplasma laidlawii. Biochim Biophys Acta. 1969 Jun 3;183(1):79–89. doi: 10.1016/0005-2736(69)90131-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mindich L. Control of fatty acid synthesis in bacteria. J Bacteriol. 1972 Apr;110(1):96–102. doi: 10.1128/jb.110.1.96-102.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindich L. Membrane synthesis in Bacillus subtilis. II. Integration of membrane proteins in the absence of lipid synthesis. J Mol Biol. 1970 Apr 28;49(2):433–439. doi: 10.1016/0022-2836(70)90255-x. [DOI] [PubMed] [Google Scholar]
- Ono Y., White D. C. Consequences of the inhibition of cardiolipin metabolism in Haemophilus parainfluenzae. J Bacteriol. 1971 Dec;108(3):1065–1071. doi: 10.1128/jb.108.3.1065-1071.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short S. A., White D. C. Biosynthesis of cardiolipin from phosphatidylglycerol in Staphylococcus aureus. J Bacteriol. 1972 Feb;109(2):820–826. doi: 10.1128/jb.109.2.820-826.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokawa Y., Nakao E., Kaziro Y. On the nature of the control by RC gene in e. coli: amino acid-dependent control of lipid synthesis. Biochem Biophys Res Commun. 1968 Oct 10;33(1):108–112. doi: 10.1016/0006-291x(68)90263-5. [DOI] [PubMed] [Google Scholar]
- THORNE K. J. THE PHOSPHOLIPIDS OF LACTOBACILLUS CASEI. Biochim Biophys Acta. 1964 Jun 15;84:350–353. doi: 10.1016/0926-6542(64)90062-9. [DOI] [PubMed] [Google Scholar]
- Thorne K. J., Constable B. J., Day K. C. Technicon autoanalysis of the ninhydrin-positive phospholipids of Lactobacillus casei. Nature. 1965 Jun 12;206(989):1156–1157. doi: 10.1038/2061156b0. [DOI] [PubMed] [Google Scholar]
- VORBECK M. L., MARINETTI G. V. SEPARATION OF GLYCOSYL DIGLYCERIDES FROM PHOSPHATIDES USING SILICIC ACID COLUMN CHROMATOGRAPHY. J Lipid Res. 1965 Jan;6:3–6. [PubMed] [Google Scholar]



