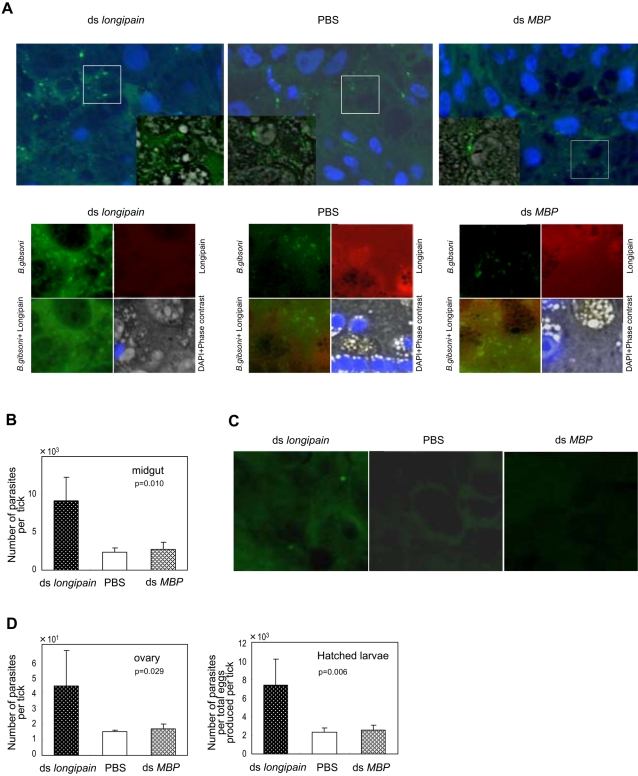
Figure 8. Knockdown of longipain by RNA interference facilitates transmission of Babesia parasites through the vector ticks.
The vectorial capacity of ds longipain RNA-treated ticks was evaluated by immunofluorescence microscopy and quantitative-PCR analysis. (A) Distribution of B. gibsoni and endogenous longipain in the midgut at 6 days of feeding. Upper panels: B. gibsoni were visualized using mouse anti-B. gibsoni antibody (green), and the nuclei of the midgut epithelial cells were stained with DAPI (blue). Areas marked by squares are shown as merged images consisting of immunofluorescence and phase contrast images at higher magnification in the corner of the original panel. Lower panels: B. gibsoni and endogenous longipain in the lumen. B. gibsoni were visualized using mouse anti-B. gibsoni antibody (green) and rabbit anti-longipain (red). Note the increased number of parasites in the lumen and the midgut epithelial cells of longipain dsRNA-injected ticks. (B) Prevalence and severity of B. gibsoni infection at day 6. The numbers of parasites invading the midgut epithelial cells were evaluated by measuring the P18 gene in the B. gibsoni genome DNA using real-time quantitative PCR. The genomic DNA was extracted from the midgut of which the lumen contents had been removed. The bars indicate means and the error bars indicate s.e.m. for three independent experiments of five ticks. (C) Representative image of migrated Babesia parasites in the ovary (×800). Parasites were detectable in the 50 serial sections of the longipain dsRNA-injected ticks but not in those from the PBS group. (D) Level of B. gibsoni infection in the ovary and eggs. The quantitative results demonstrated that repression of longipain enhanced the B. gibsoni infection in the vector tick. The bars indicate means and the error bars indicates s.e.m. for three independent experiments of four ticks.