Abstract
BACKGROUND
There is comprehensive experimental and clinical evidence that either exogenous supplementation of natural antioxidants or augmentation of endogenous antioxidants attenuates myocardial infarction.
OBJECTIVES
To investigate the effects of chronic administration of silymarin against ischemia-reperfusion-induced myocardial infarction in rats.
METHODS
Silymarin was administered orally to Wistar albino rats (200 g to 250 g) in three different doses (100 mg/kg, 250 mg/kg and 500 mg/kg), by gastric gavage for one week. At the end of this period, control (ischemia-reperfusion) groups and silymarin-treated groups were subjected to 30 min occlusion of the left anterior descending coronary artery and thereafter reperfused for 4 h.
RESULTS
Ischemia-reperfusion resulted in significant cardiac necrosis, indicated by elevated levels of serum marker enzymes such as serum glutamate oxaloacetate transaminase, serum glutamate pyruvate transaminase, lactate dehydrogenase, creatine kinase-isoenzyme and creatine kinase. A significant rise in the end products of myocardial lipid peroxides (malondialdehydes [MDAs]), loss of antioxidative enzymes (superoxide dismutase, catalase, glutathione S-transferase and reduced glutathione) and increased levels of myeloperoxidase in heart tissue were observed in the animals subjected to in vivo myocardial ischemia-reperfusion injury. Infarct size was measured by using the staining agent 2,3,5-triphenyltetrazolium chloride. A lead II electrocardiogram was monitored at various intervals throughout the experiment.
DISCUSSION
The present study showed that silymarin protected the endogenous antioxidant enzymes, suppressed the neutrophil infiltration during ischemia-reperfusion and limited the infarct size, with concomitant reduction in serum MDA, tissue MDA and serum marker enzymes in rats subjected to 30 min coronary artery occlusion followed by 4 h of reperfusion. Pretreatment with silymarin also protected rat hearts from a further drop in mean arterial blood pressure during reperfusion, and restored heart rate at the end of the reperfusion period.
CONCLUSIONS
The present study suggests that the phytochemical silymarin has cardioprotective activity against ischemia-reperfusion-induced myocardial infarction in rats.
Keywords: Infarct size, Myocardial infarction, Reperfusion injury, Silymarin
Cardiovascular disease, particularly ischemic heart disease, has become a worldwide health problem affecting all economic groups of the society. Ischemia-reperfusion injury is a major pathological factor in the natural course of ischemic heart disease (1). Myocardial ischemia-reperfusion is clinically relevant in situations such as myocardial infarction, coronary angioplasty, thrombolytic therapy, coronary revascularization and heart transplantation. The reperfusion period, although clearly beneficial for the heart, is associated with myocardial injury (2). A major goal in the management of myocardial infarction is to reduce postmyocardial infarction complications and mortality by reversing myocardial ischemia and limiting the infarct size. While much research has been done on this topic, there remains no clear understanding of the underlying causal factors associated with injury following an ischemic episode. Reperfusion can accelerate necrosis in irreversibly injured myocytes because of an increase in cell swelling disruption of cell ultrastructure, formation of contraction bands and deposition of intramitochondrial calcium phosphate granules. Sarcolemmal damage may also occur, leading to impairment of fluid regulation and influx imbalance (2–4). Several mechanisms have been proposed to explain the myocardial injury observed after ischemia and reperfusion (5). Oxidative stress is one of the mechanisms implicated in the pathogenesis of ischemic-reperfusion injury. There is comprehensive experimental and clinical evidence that antioxidants attenuate myocardial infarction (6,7). Indeed, many antioxidative plants and their isolated components have been reported to possess cardioprotective activity in experimental models of myocardial ischemia-reperfusion injury (5,8–19). Our previous studies (20) also suggested that Hydrocotyle asiatica possesses cardioprotective activity against ischemia-reperfusion injury.
Silymarin is a phytochemical isolated from the milk thistle plant Silybum marianum. Silymarin consists of a mixture of three bioflavonoids found in the fruit, seeds and leaves of the plant: silybin, silydianin and silychristin (21). Silybin is the main component (60% to 70%) and is believed to have the most biological activity. Silymarin has been shown to have utility in many liver disorders, including toxin-induced liver toxicity, hepatitis and alcoholic liver diseases (22,23). In an animal model of cirrhosis produced by bile duct obliteration, silymarin has an antifibrotic effect (24). Silymarin can prevent lipid peroxidation (25–28), inhibit low-density lipoprotein oxidation (29) and scavenge reactive oxygen species (30–32). It can also inhibit DNA synthesis (33), as well as fibroblast (34) and epidermal cell proliferation (28). Silymarin can modulate the activation of nuclear factor-kappa B to stimulate transcription (35), increase antioxidative enzyme levels (36,37) and limit lipid peroxidation (37). It can also enhance superoxide dismutase (SOD) activity and expression in vitro (38). Silymarin has been shown to increase glutathione (GS) levels in the liver by more than 50% in rats. The mechanism of action for these beneficial effects of silymarin is unknown. However, antioxidant activity is a leading theory. Hence, the aim of the present study is to evaluate the cardioprotective activity of silymarin by studying infarct size and levels of lipid peroxides, endogenous antioxidant enzymes (reduced glutathione [GSH]), antioxidant enzymes (SOD), catalase (CAT), glutathione S-transferase (GST) and serum marker enzymes, ie, lactate dehydrogenase (LDH), creatine kinase-isoenzyme (CK-MB), total CK, serum glutamate oxaloacetate transaminase (SGOT) and serum glutamate pyruvate transaminase (SGPT) against myocardial ischemia-reperfusion-induced myocardial injury in rats. The dose of silymarin that had an optimal protective effect was chosen to study the myeloperoxidase levels for assessment of neutrophil infiltration.
MATERIALS AND METHODS
Chemicals
2,3,5-triphenyltetrazolium chloride (TTC) was purchased from BDH Chemicals Ltd (England), and 1,1,3,3-tetra-ethoxypropane from Sigma Chemical Company (USA). Thiopentone sodium was supplied by Abbott Laboratories Ltd (India). Nitroblue tetrazolium, NADH, GSH, oxidized GS, 1-chloro-2,4-dinitrobenzene (CDNB), Folin phenol reagent, Ellman’s reagent and TritonX-100 were purchased from Sisco Research Laboratories Pvt Ltd (India). Phenazine methosulphate was purchased from National Chemicals (India). All other chemicals and reagents used were of analytical grade.
Animals
Albino Wistar rats (National Institute of Nutrition, India) of either sex weighing between 200 g and 250 g were selected. Animals were maintained under standard laboratory conditions at 25±2°C, relative humidity of 50±15% and normal photo-period (12 h dark and 12 h light). Commercial pellet diet (Ratan Brothers, India) and water were provided ad libitum. The experimental protocol was approved by the Institutional Animal Ethics Committee and by the animal regulatory body of the government (Regd No 516/01/A/CPCSEA).
Experimental design
The rats were divided into six groups, each consisting of five animals. Groups 1 and 2 were orally treated with 1% sodium carboxymethylcellulose, which served as a sham control and an ischemia-reperfusion control, respectively. Groups 3, 4 and 5 were treated with silymarin at doses of 100 mg/kg, 250 mg/kg and 500 mg/kg, respectively. Group 6 was treated with ramipril at a dose of 2 mg/kg, which served as a reference standard. On the eighth day, 1 h after administering the above treatments, the rats were subjected to 30 min of left anterior descending coronary artery (LAD) occlusion followed by 4 h reperfusion. The sham control group was not subjected to 30 min LAD occlusion.
Surgical procedure
Coronary artery occlusion and reperfusion was performed according to the method described by Pragada et al (39). In brief, rats were anesthetized with thiopentone sodium (30 mg/kg intraperitoneally). The neck was opened with a ventral midline incision and intubated through a tracheotomy. The animals were ventilated with room air by a Techno positive pressure respirator (Crompton Parkinson Ltd, England). Body temperature was monitored and maintained at 37°C throughout the experimental protocol. The right carotid artery was cannulated for measuring mean arterial blood pressure (MAP), and a lead II electrocardiogram (ECG) was monitored throughout the study by using Cardiart 408 (BPL) with sensitivity 20 mm/mV at a paper speed of 50 mm/s. A left thorocotomy and a pericardiotomy were performed, followed by identification of the marginal branch of the LAD. A silk thread (4-0) was passed around the artery, which was occluded by a knot for 30 min. The silk thread was removed after 30 min with the help of two-knot releasers to allow reperfusion of the heart for the succeeding 4 h.
Quantification of infarct size
In all the groups, after sacrificing the animal by injecting 2.56 M KCl directly into the left ventricle, the heart was rapidly excised from the thorax and the greater vessels were removed. The left ventricle was separated from the heart and weighed. It was sliced parallel to the atrioventricular groove into 2 mm to 3 mm thick sections and the slices were incubated in 1% TTC solution prepared in phosphate buffer (pH 7.4) for 30 min at 37°C (40). In viable myocardium, TTC is converted by dehydrogenases to a red formazan pigment that stains tissue dark red (41). Areas of the infarcted myocardium that do not take TTC stain, where the dehydrogenases are drained off, remain pale in colour. The pale necrotic tissue was separated from the stained portions and weighed on an electronic balance (Dhona 200D, Dhona Instrument Pvt Ltd, India). Infarct size was calculated as a percentage fraction of nonviable myocardium of the left ventricle.
Functional measurements
ECG changes were derived from standard II-lead ECG. After surgical preparation, rats were allowed 10 min for stabilization, and then control measurements of ECG and MAP were taken. ECG and MAP measurements were taken again before opening the thoracic cavity; before occlusion; after 15 min of occlusion; immediately after release of occlusion; and after 1 h, 2 h, 3 h and 4 h of reperfusion. Mean arterial pressure-rate product (PRP) was calculated as the product of MAP and heart rate (HR), and PRP was reported as mmHg/beats/min×103 (42).
Biochemical studies
In all the groups, before sacrificing the animal, blood samples were collected and marker enzymes such as SGOT, SGPT, LDH, CK-MB and CK in serum were estimated spectrophotometrically, using kits from Reckon Diagnostics Pvt Ltd, India, and Randox Laboratories Ltd, United Kingdom, respectively. All these marker enzymes were expressed as U/L. Serum lipid peroxide levels were estimated using the method developed by Yagi (43). Tetraethoxypropane (in amounts of 0.5 nmol, 1 nmol, 2 nmol, 4 nmol, 6 nmol, 8 nmol and 10 nmol) served as the external standard. Lipid peroxide levels in serum were expressed as μmol/L.
Estimation of protein
Protein was estimated by the Lowry method (44). The level of protein was expressed as mg protein/g tissue.
Total GSH
GSH was estimated by the method described by Ellman (45). The amount of GS was expressed as μg/g wet tissue.
GST
GST was assayed by the method described by Habig et al (46). To 0.1 mL of homogenate, 1.0 mL of 0.3 M phosphate buffer (pH 6.5), 1.7 mL of water and 0.1 mL of 30 mM CDNB were added. After incubation at 37.8°C for 15 min, 0.1 mL of GSH was added and change in optical density was read at 340 nm for 3 min at intervals of 3 s. Reaction mixture without the enzyme was used as a blank. The GST activity was expressed as nmol of CDNB conjugated/mg protein/min.
SOD
SOD level was estimated by the method described by Kakkar et al (47). The assay mixture contained 0.1 mL of sample, 1.2 mL of sodium pyrophosphate buffer (pH 8.3, 0.052 M), 0.1 mL phenazine methosulphate (186 μM), 0.3 mL of 300 μM nitroblue tetrazolium and 0.2 mL of NADH (750 μM). The reaction was started by the addition of NADH. After incubation at 30°C for 90 s, the reaction was stopped by the addition of 0.1 mL glacial acetic acid. The reaction mixture was stirred vigorously with 4.0 mL of n-butanol, and the mixture was allowed to stand for 10 min. After centrifuging the mixture, the butanol layer was separated. Colour intensity of the chromogen in the butanol was measured at 560 nm spectrophotometrically, and the concentration of SOD was expressed as U/mg protein.
CAT
CAT level was measured by the method described by Aebi (48). A volume of 0.1 mL of supernatant was added to a cuvette containing 1.9 mL of 50 mM phosphate buffer (pH 7.0). A reaction was started by the addition of 1.0 mL of freshly prepared 30 mM H2O2. The rate of decomposition of H2O2 was measured spectrophotometrically from changes in absorbance at 240 nm. Activity of CAT was expressed as μmol H2O2 metabolized/mg protein/min.
Lipid peroxide levels
Lipid peroxide levels in the myocardium were measured by the method developed by Ohkawa et al (49). Briefly, the infarcted left ventricular tissues were homogenized with 1.15% KCl (10% weight/volume). The assay mixture consisting of 0.1 mL of tissue homogenate, 0.2 mL of 8.1% sodium dodecyl sulphate, 1.5 mol of 20% acetic acid (adjusted to pH 3.5 with NaOH) and 1.5 mL of 0.8% aqueous solution of thiobarbituric acid was heated for 60 min at 95°C. Thereafter, the mixture was cooled and extracted with 5 mL of mixture of n-butanol and pyridine (15:1 volume/volume). After centrifugation at 4000 rpm for 10 min, the organic phase was assayed spectrophotometrically at 532 nm. Tetraethoxypropane (in amounts of 2 nmol, 4 nmol, 6 nmol and 8 nmol) was served as an external standard. Lipid peroxide levels in myocardium were expressed as nmol/g tissue.
Myeloperoxidase assay
Myeloperoxidase, a green hemoprotein enzyme stored in the granules of neutrophils, can use H2O2 generated by NADPH oxidase to oxidize halides (Cl−, Br− and I−) to their corresponding hypohalous acids (an additional class of active oxygen metabolite). To quantify myocardial neutrophil infiltration, the cardiac activity of myeloperoxidase, an abundant enzyme of neutriophils, was assessed using a modified method (50). Myeloperoxidase activity was used as a marker for neutrophil content in the heart, because it correlates closely with the number of neutrophils (51). The myocardial tissue was homogenized in 50 mM K2HPO4 buffer (pH 6) containing 0.5% hexadecyl-trimethylammonium bromide using a Polytron (Ultra-turrax T25, England) homogenizer. After freeze-thawing three times, the samples were centrifuged at 15,000 rpm for 30 min at 40°C, and the resulting supernatant was assayed spectrophotometrically for myeloperoxidase determination. In brief, 40 μL of sample was mixed with 960 μL of 50 mM phosphate buffer (pH 6), containing 0.167 mg/mL O-dianisidine dihydrochloride and 0.0005% H2O2. The change in absorbance at 460 nm was measured with the spectrophotometer. One unit of enzyme activity was defined as the amount of myeloperoxidase present that caused a change in absorbance measured at 460 nm for 3 min. Myeloperoxidase activity data are presented as U/g wet tissue.
Statistical analysis
The results are expressed as mean ± SEM, and the data were processed by factorial one-way ANOVA. Individual groups were compared using Dunnett’s t test. Differences with P<0.05 were considered statistically significant.
RESULTS
Effect of silymarin on myocardial infarct size and lipid peroxides
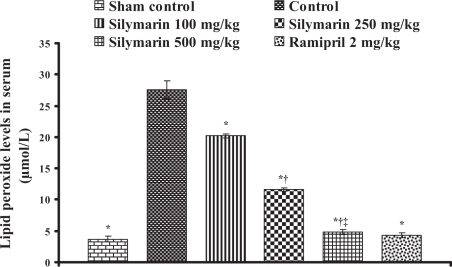
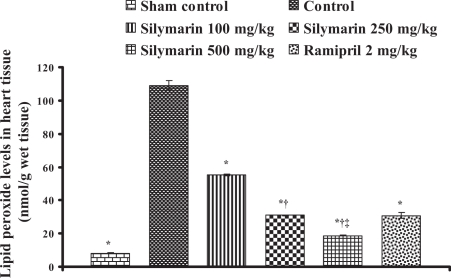
In control animals (group 2), the infarct size was found to be 50.9±1.90% and statistically significant compared with the sham control animals (group 1). In silymarin-treated animals (groups 3, 4 and 5), the infarct size was decreased to 25.26±0.78%, 13.93±0.21% and 8.28±0.43%, respectively, and in the ramipril-treated animals (group 6), the infarct size was decreased to 17.6±0.8% and the difference was statistically significant (P<0.05) compared with control (Table 1). Lipid peroxide levels in serum and heart tissue in control groups were 27.6±1.42 μmol/L (Figure 1) and 109.2±2.77 nmol/g tissue (Figure 2), respectively, and statistically significant compared with the sham control group. In groups 3, 4 and 5, the lipid peroxide levels in serum were found to be 20.18±0.33 μmol/L, 11.66±0.27 μmol/L and 4.89±0.39 μmol/L, and in heart tissue, the lipid peroxide levels were found to be 55.11±0.51 nmol/g tissue, 30.97±0.31 nmol/g tissue and 18.78±0.32 nmol/g tissue, respectively. In group 6, ramipril significantly decreased the lipid peroxide levels in serum and heart tissue, and were found to be 4.3±0.4 μmol/L and 30.8±1.9 nmol/g tissue, respectively. The results are shown in Figures 1 and 2.
TABLE 1.
Effect of silymarin on infarct size in the heart of control and experimental group animals
| Group | Dose (mg/kg) | Infarct size (% left ventricle necrosis) |
|---|---|---|
| Sham control | – | 4.50±0.79* |
| Control | – | 50.91±1.90 |
| Silymarin | 100 | 25.26±0.78* |
| Silymarin | 250 | 13.93±0.21*† |
| Silymarin | 500 | 8.28±0.43*†‡ |
| Ramipril | 2 | 17.60±0.80* |
All values are expressed as mean ± SEM. Each group consists of five albino rats.
P<0.001 compared with control group;
P<0.001 compared with silymarin 100 mg/kg group;
P<0.001 compared with silymarin 250 mg/kg group
Figure 1.
Effect of silymarin on lipid peroxide levels in serum of experimental groups. All values are expressed as mean ± SEM. Each group consists of five albino rats. *P<0.001 compared with control group; †P<0.001 compared with silymarin 100 mg/kg group; ‡P<0.01 compared with silymarin 250 mg/kg group
Figure 2.
Effect of silymarin on lipid peroxide levels in ischemic heart tissue of experimental groups. All values are expressed as mean ± SEM. Each group consists of five albino rats. *P<0.001 compared with control group; †P<0.001 compared with silymarin 100 mg/kg group; ‡P<0.01 compared with silymarin 250 mg/kg group
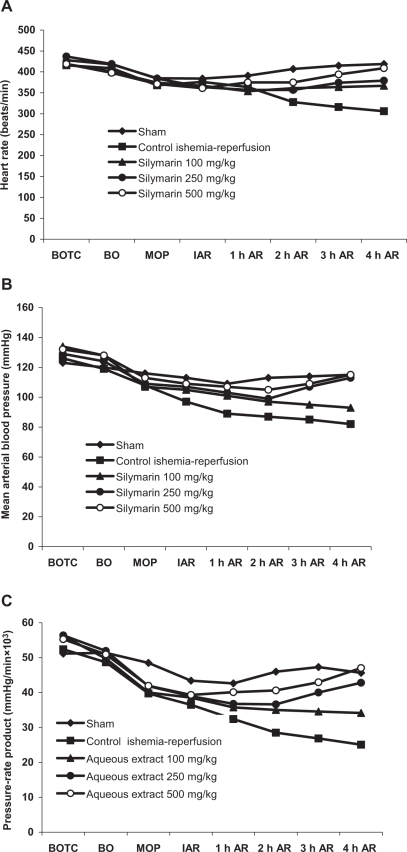
Effect of silymarin on hemodynamic variables
In the control group, sudden decreases in MAP and HR were observed after coronary artery ligation and decreased steadily throughout the occlusion and reperfusion period. Similar results were observed in calculated PRP, and the results are given in Figures 3A, 3B and 3C.
Figure 3.
Effect of silymarin at doses of 100 mg/kg, 250 mg/kg and 500 mg/kg on (A) heart rate, (B) Mean arterial blood pressure and (C) pressure-rate product in rats during occlusion of the left anterior descending coronary artery for 30 min and subsequent reperfusion for 4 h. Values are mean ± SD of five animals. AR After reperfusion; BO Before occlusion; BOTC Before opening thoracic cavity; MOP Middle of occlusion period
Effect of silymarin on myocardial antioxidants
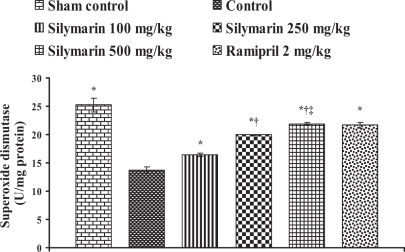
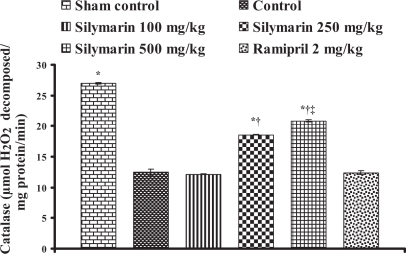
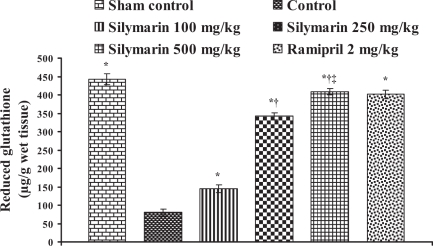
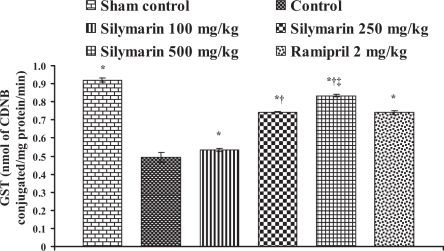
In the control group, the endogenous antioxidant enzymes such as SOD, CAT, GST and GSH levels in heart tissue were found to be 13.76±0.57 U/mg protein, 12.42±0.57 μmol H2O2 metabolized/mg protein/min, 0.494±0.026 nmol of CDNB conjugated/mg protein/min and 80.42±9.16 μg/g wet tissue, respectively, and the levels were statistically significant compared with the sham control group. In rats pretreated with silymarin at a dose of 100 mg/kg, the antioxidant enzymes levels in heart tissue were found to be 16.41±0.25 U/mg protein, 12.16±0.06 μmol H2O2 metabolized/mg protein/min, 0.534±0.01 nmol of CDNB conjugated/mg protein/min and 144.98±10.84 μg/g wet tissue, respectively. At a dose of 250 mg/kg, the antioxidant enzyme levels in heart tissue were found to be 19.99±0.07 U/mg protein, 18.57±0.08 μmol H2O2 metabolized/mg protein/min, 0.744±0.001 nmol of CDNB conjugated/mg protein/min and 342.02±9.46 μg/g wet tissue, respectively. At a dose of 500 mg/kg, the antioxidant enzymes levels were found to be 21.91±0.17 U/mg protein, 20.86±0.20 μmol H2O2 metabolized/mg protein/min, 0.834±0.006 nmol of CDNB conjugated/mg protein/min and 408.9±8.14 μg/g wet tissue, respectively, and the difference was also significant (P<0.05) compared with the control group. In group 6, ramipril significantly increased the antioxidant enzymes levels in heart tissue and were found to be 21.61±0.50 U/mg protein, 12.39±0.28 μmol H2O2 metabolized/mg protein/min, 0.74±0.01 nmol of CDNB conjugated/mg protein/min and 401.46±11.16 μg/g wet tissue, respectively (Figures 4, 5, 6 and 7).
Figure 4.
Effect of silymarin on superoxide dismutase levels in heart tissue of experimental group animals. All values are expressed as mean ± SEM. Each group consists of five albino rats. *P<0.001 compared with control group; †P<0.001 compared with silymarin 100 mg/kg group; ‡P<0.01 compared with silymarin 250 mg/kg group
Figure 5.
Effect of silymarin on catalase levels in heart tissue of experimental group animals. All values are expressed as mean ± SEM. Each group consists of five albino rats. *P<0.001 compared with control group; †P<0.001 compared with silymarin 100 mg/kg group; ‡P<0.01 compared with silymarin 250 mg/kg group
Figure 6.
Effect of silymarin on reduced glutathione levels in heart tissue of experimental group animals. All values are expressed as mean ± SEM. Each group consists of five albino rats. *P<0.001 compared with control group; †P<0.001 compared with silymarin 100 mg/kg group; ‡P<0.01 compared with silymarin 250 mg/kg group
Figure 7.
Effect of silymarin on glutathione S-transferase (GST) levels in heart tissue of experimental group animals. All values are expressed as mean ± SEM. Each group consists of five albino rats. *P<0.001 compared with control group; †P<0.001 compared with silymarin 100 mg/kg group; ‡P<0.01 compared with silymarin 250 mg/kg group. CDNB 1-chloro-2,4-dinitrobenzene
Effect of silymarin on serum marker enzymes
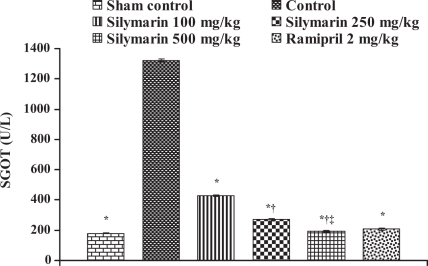
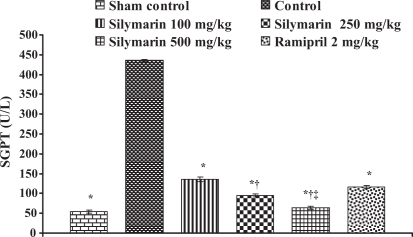
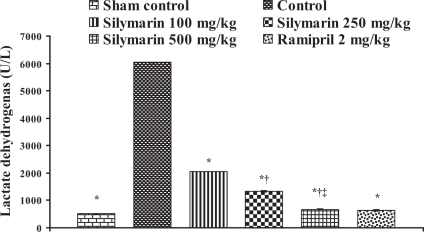
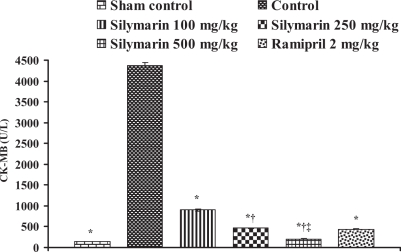
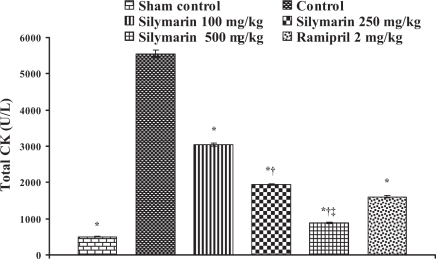
In the control group, the levels of marker enzymes such as SGOT, SGPT, LDH, CK-MB and CK in serum were found to be 1324±6.64 U/L, 435.5±3.28 U/L, 6037±6.3 U/L, 4378.66±61.23 U/L and 5551±95.19 U/L, respectively, and the levels were statistically significant compared with sham control animals. After pretreatment with silymarin at a dose of 100 mg/kg, the marker enzyme levels were found to be 428.33±4.91 U/L, 135.33±5.46 U/L, 2057±5.21 U/L, 902.66±24.36 U/L and 3045±49.38 U/L, respectively. At a dose of 250 mg/kg, the marker enzyme levels in serum were found to be 271±8.39 U/L, 94.3±3.84 U/L, 1335±23.4 U/L, 464.66±6.74 U/L and 1935±22.94 U/L, respectively. At a dose of 500 mg/kg, the marker enzyme levels were found to be 193.3±4.8 U/L, 63.33±3.84 U/L, 675.3±12.8 U/L, 204.33±5.04 U/L and 883.67±23.59 U/L, respectively, and the difference was significant (P<0.05) compared with control. In group 6, ramipril significantly decreased the marker enzyme levels in serum and were found to be 207.66±8.29 U/L, 116±3.78 U/L, 638±16.86 U/L, 437±8.50 U/L and 1605±27.3 U/L, respectively (Figures 8, 9, 10, 11 and 12).
Figure 8.
Effect of silymarin on serum glutamate oxaloacetate transaminase (SGOT) levels in serum of experimental groups. All values are expressed as mean ± SEM. Each group consists of five albino rats. *P<0.001 compared with control group; †P<0.001 compared with silymarin 100 mg/kg group; ‡P<0.01 compared with silymarin 250 mg/kg group
Figure 9.
Effect of silymarin on serum glutamate pyruvate transaminase (SGPT) levels in serum of experimental group animals. All values are expressed as mean ± SEM. Each group consists of five albino rats. *P<0.001 compared with control group; †P<0.001 compared with silymarin 100 mg/kg group; ‡P<0.01 compared with silymarin 250 mg/kg group
Figure 10.
Effect of silymarin on lactate dehydrogenase levels in serum of experimental groups. All values are expressed as mean ± SEM. Each group consists of five albino rats. *P<0.001 compared with control group; †P<0.001 compared with silymarin 100 mg/kg group; ‡P<0.01 compared with silymarin 250 mg/kg group
Figure 11.
Effect of silymarin on creatine kinase-isoenzyme (CK-MB) levels in serum of experimental group animals. All values are expressed as mean ± SEM. Each group consists of five albino rats. *P<0.001 compared with control group; †P<0.001 compared with silymarin 100 mg/kg group; ‡P<0.01 compared with silymarin 250 mg/kg group
Figure 12.
Effect of silymarin on total creatine kinase (CK) levels in serum of experimental group animals. All values are expressed as mean ± SEM. Each group consists of five albino rats. *P<0.001 compared with control group; †P<0.001 compared with silymarin 100 mg/kg group; ‡P<0.01 compared with silymarin 250 mg/kg group
Effect of silymarin on myeloperoxidase levels
In the control group, the myeloperoxidase level in heart tissue was found to be 11.68±0.18 U/g wet tissue and statistically significant compared with sham control animals (2.4±0.14 U/g wet tissue). After pretreatment with silymarin at a dose of 500 mg/kg, the myeloperoxidase level was found to be 2.48±0.26 U/g wet tissue. In the ramipril-treated (2 mg/kg) group, the myeloperoxidase level was found to be 5.96±0.33 U/g wet tissue. The results are given in Table 2.
TABLE 2.
Effect of silymarin on myeloperoxidase induction in ischemia-reperfusion-induced myocardial injury in rat hearts
| Group | Dose (mg/kg) | Myeloperoxidase (U/g tissue) | Percentage inhibition |
|---|---|---|---|
| Normal | – | 2.04±0.116 | – |
| Sham control | – | 2.4±0.141 | – |
| Control | – | 11.68±0.185 | – |
| Silymarin | 500 | 2.48±0.265* | 78.76 |
| Ramipril | 2 | 5.96±0.331* | 48.97 |
All values are expressed as mean ± SEM. Each group consists of five animals.
P<0.001 compared with the control group
DISCUSSION
As a therapeutic agent, silymarin is well tolerated and largely free of adverse effects (52). The main activity of silymarin is an antioxidant effect of its flavolignans and other polyphenolic constituents, which is attributable to radical scavenging of both free radicals and reactive oxygen species (53,54).
Recently, silymarin received attention because of its alternative beneficial activities gained indirectly from its hepatoprotective effects. These benefits include mostly anti-cancer activities, and hypocholesterolemic, anti-inflammatory and renal protective activities (55–58). These protective actions of silymarin are associated with its antioxidant properties and have been reported to act as a free radical scavenger, an inhibitor of lipid peroxidation and a plasma membrane stabilizer (59,60).
In the present investigation, we observed a significant elevation of lipid peroxide levels in serum and heart tissue, and a significant increase of infarct size in the control group, compared with the sham control group. The results clearly show the presence of cytotoxic free radical activity because there is a loss of membrane integrity, with disintegration of polyunsaturated fatty acids in the membrane bilayer, which exerts unfavourable effects on the heart structure and function. In groups treated with silymarin, the elevated lipid peroxide levels were ameliorated in both serum and heart tissue, and significantly reduced the infarct size in a dose-dependent manner, with a 500 mg/kg silymarin dose producing the greatest effect. In serum and heart tissue with the reference drug, ramipril, lipid peroxide levels decreased and significantly reduced the infarct size compared with the control group.
In the control ischemia-reperfusion group, a continuous and significant fall in MAP was observed 15 min after coronary artery occlusion and throughout the reperfusion period compared with the sham control group (Figure 3B). Pretreatment with silymarin significantly restored MAP at the end of the reperfusion period in a dose-dependent manner compared with the control group. Similarly, the HR was significantly decreased throughout the experiment duration in the control ischemia-reperfusion group compared with the 500 mg/kg group and sham control group (Figure 3A). Pretreatment with silymarin significantly restored the HR compared with the control group at the end of the reperfusion period.
The PRP was comparable for all groups before coronary artery occlusion and decreased to a similar extent immediately following occlusion (Figure 3C). Control group animals exhibited a progressively decreasing PRP during the course of the experiment, which was much lower at the end of reperfusion, compared with the value before occlusion. In contrast, treatment with silymarin significantly and dose-dependently suppressed the decrease of PRP at end of the reperfusion (Figure 3C).
Endogenous antioxidant enzymes such as SOD, CAT, GS peroxidase (GPX) and GST are the first-line cellular defense against oxidative stress, decomposing oxygen and H2O2 before they interact to form the more reactive hydroxyl radical (OH). The equilibrium between these enzymes is an important process for the effective removal of oxygen stress in intracellular organelles. SOD and CAT are important antioxidant enzymes in mitigating free radical-induced cell injury. A decrease in the activity of SOD and CAT can result in the decreased removal of superoxide ion and H2O2 radicals that brings about a number of reactions, which are harmful to myocardium. Superoxide is inactivated by SOD, the only enzyme known to use a free radical as a substrate. An increase in SOD activity is beneficial in the event of increased free radical generation. However, it has been reported that a rise in SOD activity, without a concomitant rise in the activity of CAT and/or GPX, may be detrimental because SOD generates H2O2 as a metabolite, which is more cytotoxic than oxygen radicals, and must be scavenged by CAT or GPX (61). Thus, a simultaneous increase in CAT and/or GPX activity is essential for an overall beneficial effect of an increase in SOD activity (62). In the present study, during ischemia-reperfusion injury, the levels of endogenous antioxidant enzymes (SOD, CAT, GSH and GST) in heart tissue were decreased significantly compared with sham control animals. In the treatment groups, silymarin antagonized the decrease in endogenous antioxidant enzyme levels in a dose-dependent manner and produced beneficial effects. In the ramipril-treated group, SOD, GSH and GST were protected.
Cytosolic enzymes leak into the intracellular space, where there is cell membrane damage (63,64). Increased levels of SGOT, SGPT, LDH, CK-MB and CK are well-known diagnostic markers of myocardial infarction. In the present study, marked elevations in the level of these enzymes in the serum of the control ischemia-reperfusion group suggest the occurrence of considerable membrane damage compared with the sham control group. Treatment with silymarin resulted in a significant reduction in the levels of these enzymes toward near-normal levels in a dose-dependent manner compared with the control ischemia-reperfusion group.
Because neutrophils represent a potentially important source of oxygen free radicals, myeloperoxidase, an enzyme that is associated with the azurophilic granules of neutrophils, has been measured to form an index of the infiltration of neutrophils into inflamed tissue. In the present study, the myeloperoxidase levels are significantly elevated in the control group compared with the sham control group. In silymarin-treated groups, the reduced levels of myeloperoxidase indicated that pretreatment with silymarin suppressed neutrophil infiltration into the injured myocardium.
The present findings suggest that silymarin, a well-known liver protectant and phytochemical, posseses dose-dependent cardioprotection against ischemia-reperfusion-induced myocardial injury. Reduction in infarct size, as well as decreased levels of lipid peroxides in serum and heart tissue, clearly indicates that silymarin neutralizes the cytotoxic free radicals generated during ischemia-reperfusion injury, thereby protecting against the loss of membrane integrity and stabilizing the membrane. Hence, silymarin protects myocytes from oxidative stress. This is further confirmed by the investigations on antioxidant enzymes (SOD, CAT, GST and GSH) and serum marker enzymes (SGOT, SGPT, LDH, CK-MB and CK), which leak out from tissues to plasma when degenerative changes develop in myocardial cell membranes. Suppressing the neutrophil infiltration and preventing the fall in mean arterial pressure and HR during ischemia-reperfusion further support the protection offered by silymarin against ischemia-reperfusion injury.
REFERENCES
- 1.Hansen PR. Myocardial reperfusion injury: Experimental evidence and clinical relevance. Eur Heart J. 1995;16:734–40. doi: 10.1093/oxfordjournals.eurheartj.a060991. [DOI] [PubMed] [Google Scholar]
- 2.Ferrari R, Alfieri O, Curello S, et al. Occurrence of oxidative stress during reperfusion of the human heart. Circulation. 1990;81:201–11. doi: 10.1161/01.cir.81.1.201. [DOI] [PubMed] [Google Scholar]
- 3.Bernier M, Hearse DJ, Manning AS. Reperfusion-induced arrhythmias and oxygen-derived free radicals. Studies with “anti-free radical” interventions and a free radical-generating system in the isolated perfused rat heart. Circ Res. 1986;58:331–40. doi: 10.1161/01.res.58.3.331. [DOI] [PubMed] [Google Scholar]
- 4.Curello S, Ceconi C, Medici D, Ferrari R. Oxidative stress during myocardial ischemia and reperfusion: Experimental and clinical evidences. J Appl Cardiol. 1986;1:311–27. [Google Scholar]
- 5.Mohanty I, Singh Arya D, Dinda A, Joshi S, Talwar KK, Gupta SK. Protective effects of Curcuma longa on ischemia-reperfusion induced myocardial injuries and their mechanisms. Life Sci. 2004;75:1701–11. doi: 10.1016/j.lfs.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 6.Hearse DJ. Prospects for antioxidant therapy in cardiovascular medicine. Am J Med. 1991;91:118S–21S. doi: 10.1016/0002-9343(91)90294-8. [DOI] [PubMed] [Google Scholar]
- 7.Bernier M, Manning AS, Hearse DJ. Reperfusion arrhythmias: Dose-related protection by anti-free radical interventions. Am J Physiol. 1989;256:H1344–H52. doi: 10.1152/ajpheart.1989.256.5.H1344. [DOI] [PubMed] [Google Scholar]
- 8.Ahmed KKM, Rana AC, Dixit VK. Effect of Calotropis procera latex on isoproterenol induced myocardial infarction in albino rats. Phytomedicine. 2004;11:327–30. doi: 10.1078/0944711041495146. [DOI] [PubMed] [Google Scholar]
- 9.Manikandan P, Sumitra M, Aishwarya S, Manohar BM, Lokanadam B, Puvanakrishnan R. Curcumin modulates free radical quenching in myocardial ischemia in rats. Int J Biochem Cell Biol. 2004;36:1967–80. doi: 10.1016/j.biocel.2004.01.030. [DOI] [PubMed] [Google Scholar]
- 10.Karthikeyan K, Bai BR, Gauthaman K, Sathish KS, Devaraj SN. Cardioprotective effect of the alcoholic extract of Terminalia arjuna bark in an in vivo model of myocardial ischemia reperfusion injury. Life Sci. 2003;73:2727–39. doi: 10.1016/s0024-3205(03)00671-4. [DOI] [PubMed] [Google Scholar]
- 11.Saravanan G, Prakash J. Effect of garlic (Allium sativum) on lipid peroxidation in experimental myocardial infarction in rats. J Ethnopharmacol. 2004;94:155–8. doi: 10.1016/j.jep.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 12.Senthil kumar SH, Anandan R, Devaki T, Santhosh Kumar M. Cardioprotective effects of Picrorrhiza kurroa against isoproterenol-induced myocardial stress in rats. Fitoterapia. 2001;72:402–5. doi: 10.1016/s0367-326x(01)00264-7. [DOI] [PubMed] [Google Scholar]
- 13.Kurian GA, Philip S, Varghese T. Effect of aqueous extract of Desmodium gangeticum DC root in the severity of myocardial infarction. J Ethnopharmacol. 2005;97:457–61. doi: 10.1016/j.jep.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 14.Gauthaman K, Maulik M, Kumari R, Manchanda SC, Dinda AK, Maulik SK. Effect of chronic treatment with bark of Terminalia arjuna: A study on the isolated ischemic-reperfused rat heart. J Ethnopharmacol. 2001;75:197–201. doi: 10.1016/s0378-8741(01)00183-0. [DOI] [PubMed] [Google Scholar]
- 15.Nirmala C, Puvanakrishnan R. Protective role of curcumin against isoproterenol induced myocardial infarction in rats. Mol Cell Biochem. 1996;159:85–93. doi: 10.1007/BF00420910. [DOI] [PubMed] [Google Scholar]
- 16.Sharma M, Kishore K, Gupta SK, Joshi S, Arya DS. Cardioprotective potential of Ocimum sanctum in isoproterenol induced myocardial infarction in rats. Mol Cell Biochem. 2001;225:75–83. doi: 10.1023/a:1012220908636. [DOI] [PubMed] [Google Scholar]
- 17.Gupta SK, Mohanty I, Talwar KK, et al. Cardioprotection from ischemia and reperfusion injury by Withania somnifera: A hemodynamic, biochemical and histopathological assessment. Mol Cell Biochem. 2004;260:39–47. doi: 10.1023/b:mcbi.0000026051.16803.03. [DOI] [PubMed] [Google Scholar]
- 18.Sumitra M, Manikandan P, Kumar DA, et al. Experimental myocardial necrosis in rats: Role of arjunolic acid on platelet aggregation, coagulation and antioxidant status. Mol Cell Biochem. 2001;224:135–42. doi: 10.1023/a:1011927812753. [DOI] [PubMed] [Google Scholar]
- 19.Suchalatha S, Shyamala Devi CS. Protective effect of Terminalia chebula against experimental myocardial injury by isoproterenol. Indian J Exp Biol. 2004;42:174–8. [PubMed] [Google Scholar]
- 20.Pragada RR, Veeravalli KK, Chowdary KP, Routhu KV. Cardioprotective activity of Hydrocotyle asiatica L. in ischemia-reperfusion induced myocardial infarction in rats. J Ethnopharmacol. 2004;93:105–8. doi: 10.1016/j.jep.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 21.Pepping J. Milk thistle: Silybum marianum. Am J Health System Pharm. 1999;56:1195–7. doi: 10.1093/ajhp/56.12.1195. [DOI] [PubMed] [Google Scholar]
- 22.Ferenci P, Dragosics B, Dittrich H, et al. Randomized controlled trail of silymarin treatment in patients with cirrhosis of the liver. J Hepatol. 1989;9:105–13. doi: 10.1016/0168-8278(89)90083-4. [DOI] [PubMed] [Google Scholar]
- 23.Salmi HA, Sarna S. Effect of silymarin on chemical, functional, and morphological alterations of the liver. A double-blind controlled study. Scand J Gastroenterol. 1982;17:517–21. doi: 10.3109/00365528209182242. [DOI] [PubMed] [Google Scholar]
- 24.Boigk G, Stroedter L, Herbst H, Waldschmidt J, Riecken EO, Schuppan D. Silymarin retards collagen accumulation in early and advanced biliary fibrosis secondary to complete bile duct obliteration in rats. Hepatology. 1997;26:643–9. doi: 10.1002/hep.510260316. [DOI] [PubMed] [Google Scholar]
- 25.Carini R, Comoglio A, Albano E, Poli G. Lipid peroxidation and irreversible damage in the rat hepatocyte model. Protection by the silybin-phosholipid complex IdB 1016. Biochem Pharmacol. 1992;43:2111–5. doi: 10.1016/0006-2952(92)90168-i. [DOI] [PubMed] [Google Scholar]
- 26.Velussi M, Cernigoi AM, De Monte A, Dapas F, Caffau C, Zilli M. Long-term (12 months) treatment with an anti-oxidant drug (silymarin) is effective on hyperinsulinemia, exogenous insulin need and malondialdehyde levels in cirrhotic diabetic patients. J Hepatol. 1997;26:871–9. doi: 10.1016/s0168-8278(97)80255-3. [DOI] [PubMed] [Google Scholar]
- 27.Chatterjee ML, Agarwal R, Mukhtar H. Ultraviolet B radiation-induced DNA lesions in mouse epidermis: An assessment using a novel 32P-postlabelling technique. Biochem Biophys Res Commun. 1996;229:590–5. doi: 10.1006/bbrc.1996.1848. [DOI] [PubMed] [Google Scholar]
- 28.Lahiri-Chatterjee M, Katiyar SK, Mohan RR, Agarwal R. A flavonoid antioxidant silymarin, affords exceptionally high protection against tumor promotion in the SENCAR mouse skin tumorigenesis model. Cancer Res. 1999;59:622–32. [PubMed] [Google Scholar]
- 29.Skottová N, Krecman V, Simánek V. Activities of silymarin and its flavonolignans upon low density lipoprotein oxidizability in vitro. Phytotherapy Res. 1999;13:535–7. doi: 10.1002/(sici)1099-1573(199909)13:6<535::aid-ptr526>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 30.Dehmlow C, Erhard J, de Groot H. Inhibition of Kupffer cell functions as an explanation for the hepatoprotective properties of silibinin. Hepatology. 1996;23:749–54. doi: 10.1053/jhep.1996.v23.pm0008666328. [DOI] [PubMed] [Google Scholar]
- 31.Dehmlow C, Murawski N, de Groot H. Scavenging of reactive oxygen species and inhibition of arachidonic acid metabolism by silibinin in human cells. Life Sci. 1996;58:1591–600. doi: 10.1016/0024-3205(96)00134-8. [DOI] [PubMed] [Google Scholar]
- 32.Mira L, Silva M, Manso CF. Scavenging of reactive oxygen species by silibinin dihemisuccinate. Biochem Pharmacol. 1994;48:753–9. doi: 10.1016/0006-2952(94)90053-1. [DOI] [PubMed] [Google Scholar]
- 33.Ahmad N, Gali H, Javed S, Agarwal R. Skin cancer chemopreventive effects of a flavonoid antioxidant silymarin are mediated via impairment of receptor tyrosine kinase signaling and pertubation in cell cycle progression. Biochem Biophys Res Commun. 1998;247:294–301. doi: 10.1006/bbrc.1998.8748. [DOI] [PubMed] [Google Scholar]
- 34.Onat D, Boscoboinik D, Azzi A, Basaga H. Effect of alpha-tocopherol and silybin dihemisuccinate on the proliferation of human skin fibroblasts. Biotechnol Appl Biochem. 1999;29:213–5. [PubMed] [Google Scholar]
- 35.Saliou C, Kitazawa M, McLaughlin L, et al. Antioxidants modulate acute solar ultraviolet radiation-induced NF-kappa-B activation in a human keratinocyte cell line. Free Radic Biol Med. 1999;26:174–83. doi: 10.1016/s0891-5849(98)00212-3. [DOI] [PubMed] [Google Scholar]
- 36.Soto C, Recoba R, Barrón H, Alvarez C, Favari L. Silymarin increases antioxidant enzymes in alloxan-induced diabetes in rat pancreas. Comp Biochem Physiol C Toxicol Pharmacol. 2003;136:205–12. doi: 10.1016/s1532-0456(03)00214-x. [DOI] [PubMed] [Google Scholar]
- 37.Müzes G, Deák G, Láng I, Nékám K, Niederland V, Fehér J. Effect of silymarin (Legalon) therapy on the antioxidant defense mechanism and lipid peroxidation in alcoholic liver disease (double blind protocol) Orv Hetil. 1990;131:863–6. [PubMed] [Google Scholar]
- 38.Láng I, Deák G, Müzes G, Prónai L, Fehér J. Effect of the natural bioflavonoid antioxidant silymarin on superoxide dismutase (SOD) activity and expression in vitro. Biotechnol Ther. 1993;4:263–70. [PubMed] [Google Scholar]
- 39.Rao PR, Kumar VK, Viswanath RK, Subbaraju GV. Cardioprotective activity of alcoholic extract of Tinospora cordifolia in ischemia-reperfusion induced myocardial infarction in rats. Biol Pharm Bull. 2005;28:2319–22. doi: 10.1248/bpb.28.2319. [DOI] [PubMed] [Google Scholar]
- 40.Johnson G, 3rd, Tsao P, Lefer AM. Synergism between superoxide dismutase and sodium nitrate in cardioprotection following ischemia and reperfusion. Am Heart J. 1990;119:530–8. doi: 10.1016/s0002-8703(05)80275-3. [DOI] [PubMed] [Google Scholar]
- 41.Fishbein MC, Meerbaum S, Rit J, et al. Early phase acute myocardial infarct size quantification: Validation of triphenyl tetrazolium chloride tissue enzyme staining technique. Am Heart J. 1981;101:593–600. doi: 10.1016/0002-8703(81)90226-x. [DOI] [PubMed] [Google Scholar]
- 42.Gobel FL, Norstrom LA, Nelson RR, Jorgensen CR, Wang Y. The rate-pressure product as an index of myocardial oxygen consumption during exercise in patients with angina pectoris. Circulation. 1978;57:549–56. doi: 10.1161/01.cir.57.3.549. [DOI] [PubMed] [Google Scholar]
- 43.Yagi K. A simple fluorometric assay for lipoperoxide in blood plasma. Biochem Med. 1976;15:212–6. doi: 10.1016/0006-2944(76)90049-1. [DOI] [PubMed] [Google Scholar]
- 44.Lowry OH, Rosebrough NJ, Farr AL, Randal RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 45.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 46.Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–9. [PubMed] [Google Scholar]
- 47.Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21:130–2. [PubMed] [Google Scholar]
- 48.Aebi H. Catalase. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. Academic Press; New York: 1974. pp. 673–84. [Google Scholar]
- 49.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 50.Mullane KM, Kraemer R, Smith B. Myeloperoxidase activity as a quantitative assessment of neutrophil infiltration into ischemic myocardium. J Pharmacol Methods. 1985;14:157–67. doi: 10.1016/0160-5402(85)90029-4. [DOI] [PubMed] [Google Scholar]
- 51.Weyrich AS, Ma XL, Lefer AM. The role of L-arginine in ameliorating reperfusion injury after myocardial ischemia in the cat. Circulation. 1992;86:279–88. doi: 10.1161/01.cir.86.1.279. [DOI] [PubMed] [Google Scholar]
- 52.Morazzoni P, Bombardelli E. Silybum marianum (Carduus marianus) Fitoterapia. 1995;66:3–42. [Google Scholar]
- 53.Valenzuela A, Guerra R, Videla LA. Antioxidant properties of the flavonoids silybin and (+)-cyanidanol-3: Comparison with butylated hydroxyanisole and butylated hydroxytoluene. Planta Med. 1986;52:438–40. doi: 10.1055/s-2007-969247. [DOI] [PubMed] [Google Scholar]
- 54.Comoglio A, Leonarduzzi G, Carini R, et al. Studies on the antioxidant and free radical scavenging properties of IdB 1016 a new flavanolignan complex. Free Radic Res Commun. 1990;11:109–15. doi: 10.3109/10715769009109673. [DOI] [PubMed] [Google Scholar]
- 55.Skottová N, Krecman V. Dietary silymarin improves removal of low density lipoproteins by the perfused rat liver. Acta Univ Palacki Olomuc Fac Med. 1998;141:39–40. [PubMed] [Google Scholar]
- 56.Skottová N, Krecman V. Silymarin as a potential hypocholesterolaemic drug. Physiol Res. 1998;47:1–7. [PubMed] [Google Scholar]
- 57.Kang JS, Jeon YJ, Park SK, Yang KH, Kim HM. Protection against lipopolysaccharide-induced sepsis and inhibition of interleukin-1beta and prostaglandin E2 synthesis by silymarin. Biochem Pharmacol. 2004;67:175–81. doi: 10.1016/j.bcp.2003.08.032. [DOI] [PubMed] [Google Scholar]
- 58.Sanhueza J, Valdes J, Campos R, Garrido A, Valenzuela A. Changes in the xanthine dehydrogenase/xanthine oxidase ratio in the rat kidney subjected to ischemia-reperfusion stress: Preventive effect of some flavonoids. Res Commun Chem Pathol Pharmacol. 1992;78:211–8. [PubMed] [Google Scholar]
- 59.Muriel P, Garciapiña T, Perez-Alvarez V, Mourelle M. Silymarin protects against paracetamol-induced lipid peroxidation and liver damage. J Appl Toxicol. 1992;12:439–42. doi: 10.1002/jat.2550120613. [DOI] [PubMed] [Google Scholar]
- 60.Haková H, Misúrová E, Kropácová K. The effect of silymarin on concentration and total content of nucleic acids in tissues of continuously irradiated rats. Vet Med (Praha) 1996;41:113–9. [PubMed] [Google Scholar]
- 61.Yim MB, Chock PB, Stadtman ER. Copper, zinc superoxide dismutase catalyzes hydroxy radical production from hydrogen peroxide. Proc Natl Acad Sci U S A. 1990;87:5006–10. doi: 10.1073/pnas.87.13.5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harman D. The aging process: Major risk factor for disease and death. Proc Natl Acad Sci U S A. 1991;88:5360–3. doi: 10.1073/pnas.88.12.5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mueller EA, Griffin WS, Wildenthal K. Isoproterenol-induced cardiomyopathy: Changes in cardiac enzymes and protection by methylprednisolone. J Mol Cell Cardiol. 1977;9:565–78. doi: 10.1016/s0022-2828(77)80371-4. [DOI] [PubMed] [Google Scholar]
- 64.Lombardini JB. Effects of isoproterenol and methoxamine on the contents of taurine in rat tissues. J Pharmacol Exp Ther. 1980;213:399–405. [PubMed] [Google Scholar]