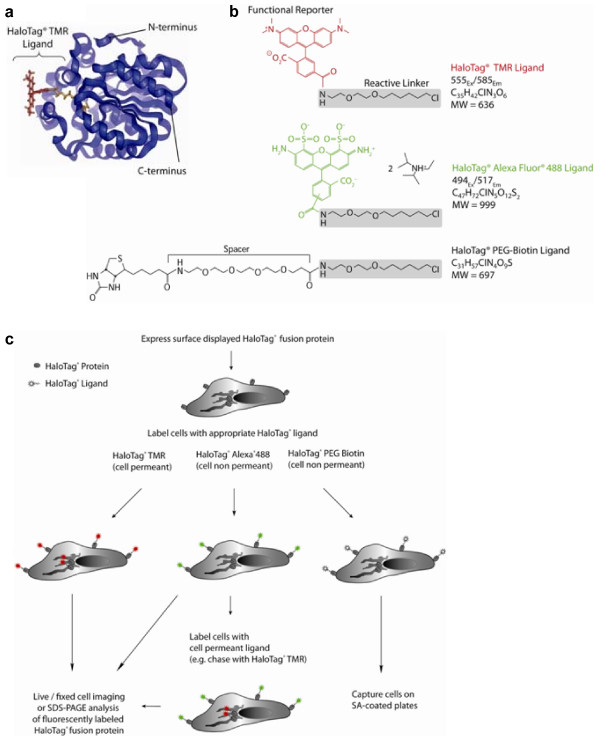
Figure 1.
Overview of HaloTag® Technology. (a) Molecular model of the HaloTag protein. The HaloTag TMR ligand (fluorescent moiety in red, reactive linker in orange) is shown covalently bound to the aspartate nucleophile (blue). Replacement of catalytic base (His) with Phe renders the HaloTag protein inactive, leading to the formation of a stable covalent bond [13]. (b) Chemical structure of the HaloTag ligands showing the functional reporters and the reactive linker. HaloTag ligands have the same chloroalkane reactive linker, but differ in the functional reporter and distance of the reporter from the linker. The HaloTag TMR ligand crosses the cell membrane, unlike the HaloTag PEG-Biotin and Alexa 488 Ligands. (c) The interchangeable HaloTag technology permits several cell-based applications, including live or fixed cell imaging and SDS-page analysis. Abbreviations: TMR, tetramethyl-rhodamine; His, histidine; Phe, phenylalanine.